ATOMIC THEORY
1/30
Earn XP
Description and Tags
LESSON 3
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
ATOMOS
“indivisible” or “uncuttable”
DEMOCRITUS (5th century B.C)
All matter is composed of small, finite particles and called it atomos. Atoms as moving particles that differed in shape and size, could join together.
ARISTOTLE (5th century B.C)
Matter is consisted of various combinations of the four “elements”— fire, earth, air, and water
ATOMS
Each element is composed of extremely small particles.
JOHN DALTON (19th Century)
Each element is composed of extremely small particles called atoms.
J.J. THOMSON
observed that cathode rays are the same regardless of the identity of the cathode material. Discovered electrons.
CATHODE RAYS
are streams of negatively charged particles and these negatively charged particles are called electrons.
ROBERT MILLIKAN
succeeded in measuring the charge of an electron by performing the oil-drop experiment in 1909.
ELECTRON CHARGE
1.602 x 10-9 C
RADIOACTIVITY
spontaneous emission of radiation.
RADIATION
the emission of energy as electromagnetic waves or as moving subatomic particles, especially high-energy particles that cause ionization.
HENRI BECQUEREL
discovered that a compound of uranium spontaneously emits high-energy radiation in 1896.
MARIE AND PIERRE CURIE
began experiments to identify and isolate the source of radioactivity in the compound at Becquerel’s suggestion.
ERNEST RUTHERFORD
revealed three types of radiation: alpha α, beta β, and gamma γ. Discoverd proton and nucelus
PROTON
positive charge
NEUTRON
uncharge
ELECTRON
negative charge
NUCLEUS
known to contain almost all of the mass of an atom, with the number of protons only providing half, or less, of that mass.
MESOTHORIUM
a “new element” produced by the radioactive decay of thorium
FREDERICK SODDY
discovered an element could have types of atoms with different masses that were chemically indistinguishable. And he called these isotopes
ISOTOPES
atoms of the same element that differ in mass.
JAMES CHADWICK
discovered neutrons: uncharged, subatomic particles with a mass approximately the same as that of protons
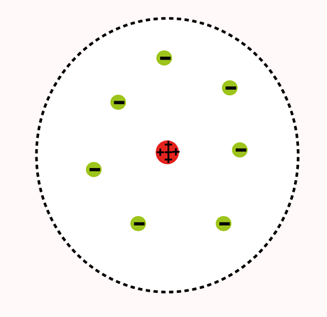
DALTON’S ATOMIC MODEL

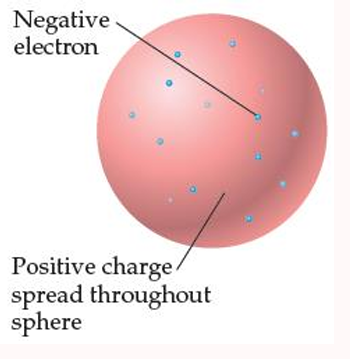
J.J. THOMSON’S PLUM-PUDDING MODEL
The atom consists of a uniform positive sphere of matter in which the mass is evenly distributed and in which the electrons are embedded like raisins in a pudding or seeds in a watermelon
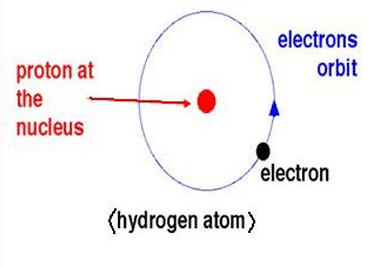
RUTHERFORD’S NUCLEAR MODEL
most of the volume of an atom is empty space in which electrons move around the nucleus. Modern era’s view of the atom is a miniature “solar system”
NIELS BOHR
attempted to resolve the atomic paradox by ignoring classical electromagnetism’s prediction that the orbiting electron in hydrogen would continuously emit light.
BOHR’S MODEL
assumed that the electron orbiting the nucleus would not normally emit any radiation (the stationary state hypothesis), but it would emit or absorb a photon if it moved to a different orbit.
LOUIS DE BROGLIE
one of the first people to pay attention to the special behavior of the microscopic world. “λ = h/mv”
WERNER HEISENBERG
considered the limits of how accurately we can measure properties of an electron or other microscopic particles. He determined that there is a fundamental limit to how accurately one can measure both a particle’s position and its momentum simultaneously.
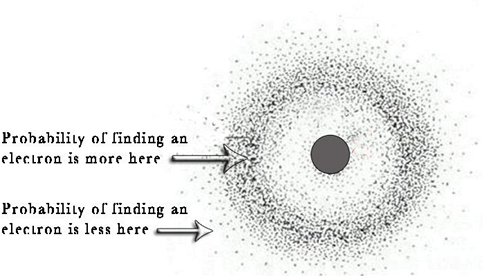
ERWIN SCHRODINGER
proposed an equation that incorporates both the wave like and particle-like behaviors of the electron (Schrödinger equation). 𝐇𝛙=E𝛙. Discovered quantum mechanics or wave mechanics
SCHRODINGER’S MODEL