12. SNAREs 2: Membrane fusion machinery in health and disease
1/49
Earn XP
Description and Tags
complete
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
What happens when membrane fusion is inhibited in animals?
Disrupts essential processes like neurotransmission, secretion, and vesicle trafficking, leading to severe physiological and behavioural impairments, including paralysis and, in some cases, lethality.
What is the shibire mutation in flies, and how does it affect membrane fusion?
Affects dynamin, a protein necessary for vesicle scission during endocytosis.
Causes temperature-sensitive paralysis in flies due to the inability to recycle synaptic vesicles, thus inhibiting neurotransmitter release.
What is the comatose mutation in flies, and what does it affect?
Affects NSF, a protein crucial for recycling SNARE complexes after fusion.
Results in temperature-sensitive paralysis due to the failure of neurotransmitter vesicle recycling and membrane fusion.
What does the paralytic mutation affect, and how does it relate to membrane fusion?
Affects the α-subunit of the voltage-gated sodium channel, essential for action potential propagation in neurons.
Impairs the electrical signals that trigger SNARE-mediated vesicle fusion, leading to paralysis in flies.
What happens to docked vesicles in comatose flies at the restrictive temperature?
In comatose flies, which have a mutation in NSF, docked vesicles accumulate at the synapse when exposed to the restrictive temperature.
This occurs because NSF is unable to disassemble SNARE complexes, preventing vesicles from being recycled for subsequent rounds of neurotransmitter release, leading to a build-up of vesicles ready to fuse but unable to complete the fusion process.
What happens to mice with a VAMP2 gene knockout, and what does this reveal about VAMP2’s function?
Die at birth and show a loss of synaptic transmission, indicating that VAMP2 is essential for neurotransmitter release and viability.
What phenotype is observed in mice with a Syntaxin1A gene knockout?
No gross abnormalities but display subtle defects in synaptic transmission, suggesting that while Syntaxin1A is important for synaptic function, it may be partially compensated by other proteins.
What is the effect of knocking out the Syntaxin1B gene in mice?
Die shortly after birth and exhibit reduced synaptic transmission, highlighting the crucial role of Syntaxin1B in maintaining early-life neural function.
What phenotype is observed in SNAP25 knockout mice, and what does it imply about SNAP25’s role?
Die at birth and show a loss of synaptic transmission, indicating that SNAP25 is essential for neurotransmission and survival.
What disorder is associated with mutations in the VAMP2 gene, and what are its symptoms?
Linked to a neurodevelopmental disorder characterized by hypotonia, autistic features, and sometimes hyperkinetic movements, affecting motor and social development.
What are the effects of SNAP25b mutations in humans?
A neurodevelopmental disorder with symptoms including seizures, intellectual disability, severe speech delay, and cerebellar ataxia, impacting cognitive and motor functions.
What is CEDNIK syndrome, and which SNARE gene mutation causes it?
Caused by mutations in the SNAP29 gene, leading to cerebral dysgenesis, neuropathy, ichthyosis, and palmoplantar keratoderma, affecting skin, neurological development, and motor function.
Which disorder is caused by Syntaxin 11 mutations, and what are its primary characteristics?
Familial Hemophagocytic Lymphohistiocytosis Type 4 (FHL4), a rare immune disorder characterized by uncontrolled immune activation leading to severe inflammation.
What is the impact of heterozygous mutations in VAMP2, and what is a common symptom from birth?
Cause a severe neurodevelopmental disorder, with all affected individuals experiencing hypotonia (low muscle tone) from birth. This condition affects motor development and often involves other neurological symptoms.
Why are there relatively few diseases associated with SNARE protein mutations?
Many of them have redundant isoforms or backup mechanisms that can partially compensate for dysfunction.
For example, VAMP2 and SNAP25b are not the main isoforms, so mutations in these specific isoforms are less likely to result in widespread disease.
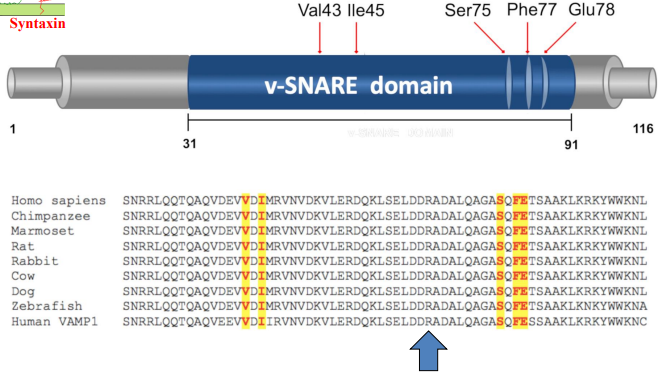
Where do mutations associated with neurodevelopmental disorders map within the VAMP2 protein?
Map to the SNARE domain of the protein, which is essential for vesicle fusion. These mutations involve specific amino acid changes that disrupt the SNARE-mediated membrane fusion process critical for synaptic transmission and cellular communication.
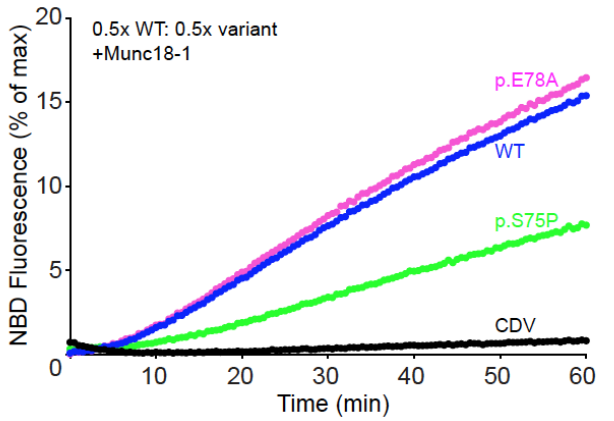
What is the effect of the S75P mutation in VAMP2, and how does it compare to the wild-type and p.E78A mutation?
The S75P mutation in VAMP2 is a dominant negative mutation, meaning it interferes with normal protein function even in the presence of a wild-type allele. This mutation shows a slower increase in NBD fluorescence over time compared to both the wild-type, indicating impaired SNARE complex assembly and slower membrane fusion dynamics.
S75P slows the rate of liposome fusion.
What is Familial Hemophagocytic Lymphohistiocytosis (FHL)?
A rare and life-threatening immune disorder primarily affecting infants.
Characterized by uncontrolled immune activation, leading to excessive proliferation of T cells, natural killer cells, B cells, and macrophages.
What serious complication can occur in Familial Hemophagocytic Lymphohistiocytosis (FHL), and why is it dangerous?
Can cause a cytokine storm, a dangerous condition where excessive cytokine release triggers severe inflammation, which can lead to organ damage and is potentially life-threatening.
What are some genetic causes of Familial Hemophagocytic Lymphohistiocytosis?
Caused by mutations in several genes, including those involved in immune cell function, such as Syntaxin 11.
Mutations impair the ability of immune cells to regulate inflammation effectively.
Why are FHL patients at risk of dying from infections?
Defective cytotoxic activity in T cells, meaning they are unable to kill infected cells effectively, leading to an increased risk of severe infections.
How do T cells kill infected cells?
By secreting cytotoxic granules containing proteins like perforin and granzymes. Perforin forms pores in the target cell's membrane, allowing granzymes to enter and induce apoptosis, effectively eliminating the infected cell.
What mutation is associated with Familial Hemophagocytic Lymphohistiocytosis Type 4 (FHL4)?
Mutations in the STX11 gene cause Familial Hemophagocytic Lymphohistiocytosis Type 4 (FHL4). STX11 is an unusual Q-SNARE that lacks a transmembrane domain, affecting its function in immune response.
How does STX11 deficiency impact patients with FHL4?
Significantly reduced levels of STX11, leading to impaired immune function. This deficiency is linked to defective degranulation from cytotoxic T-cells, although the exact mechanism of this impairment remains unclear.
What is the role of STX11 in T-cell function?
Docking and fusion of cytotoxic granules with the plasma membrane in T-cells, facilitating the release of cytotoxic proteins necessary for targeting and destroying infected cells.
What mutation is associated with Familial Hemophagocytic Lymphohistiocytosis Type 5 (FHL5), and how does it affect STX11 levels?
Mutations in Munc18-2.
Reduced levels of STX11 - impairing function of cytotoxic T-cells and contributing to the immune dysregulation seen in FHL5.
What disease is caused by Clostridium tetani, and what is a common symptom?
Tetanus, commonly known as lockjaw. It is characterized by severe muscle stiffness and spasms, particularly in the jaw and neck.
How many people die from tetanus each year globally?
~50,000 people, despite the availability of vaccines.
What condition is caused by Clostridium botulinum?
Botulism, a serious illness that can lead to paralysis and is often associated with improperly canned foods.
What is the incidence rate of botulism each year?
Around 100-200 people are reported each year in the United States.
How potent are Clostridial neurotoxins, and what is their LD50?
Clostridial neurotoxins are among the most potent biological toxins known, with an LD50 of 1-2 ng/kg, meaning that incredibly small amounts can be lethal.
LD50 = the amount of an ingested substance that kills 50 percent of a test sample
Which age groups are most affected by global deaths from tetanus?
Predominantly occur in children under the age of 5, followed by individuals aged 70 and older.
Deaths then descend in an order of older to younger with the least deaths seen in the 5-14 year age group.
Why do certain age groups experience higher global deaths from tetanus?
Children under 5 years are at higher risk for tetanus due to incomplete vaccination and susceptibility to infections from wounds.
Individuals aged 70 and older may also have reduced immunity and health complications that make them more vulnerable.
In contrast, older children and young adults often have better vaccination coverage and overall health, resulting in fewer deaths from tetanus.
What is the most common form of botulism?
Infant botulism, Babies under 6 months are the most susceptible due to immature gastrointestinal systems.
Hallmark symptom = floppy baby syndrome, characterized by weakness, poor muscle tone, and difficulty feeding.
What are the different domains of clostridial neurotoxins?
Targeting Domain - Directs the toxin to specific neuronal receptors.
Translocation Domain - Facilitates the entry of the toxin into the cytosol of the neuron.
Protease Domain - Responsible for the enzymatic activity that cleaves proteins involved in neurotransmitter release.
What is the enzymatic function of clostridial neurotoxins?
Classified as zinc-dependent proteases, meaning they utilize zinc ions to catalyse the cleavage of specific proteins, particularly those in the synaptic vesicle release machinery.
What type of cells take up clostridial toxins?
Specifically taken up by neurons, where they exert their neurotoxic effects by interfering with neurotransmitter release.
What is the significance of SNARE protein cleavage by BoNT?
Disrupts the formation of the SNARE complex, preventing the docking and fusion of synaptic vesicles with the presynaptic membrane, ultimately leading to inhibition of neurotransmitter release and muscle paralysis.
How do clostridial neurotoxins affect the neuromuscular junction?
Inhibit the release of acetylcholine at the neuromuscular junction. By cleaving SNARE proteins necessary for synaptic vesicle fusion, these toxins prevent the release of acetylcholine, leading to muscle paralysis and loss of voluntary movement.
How are tetanus and botulinum toxins similar in mode of action but target different neurons for intoxication?
Tetanus and botulinum toxins are both zinc-dependent proteases that cleave SNARE proteins, disrupting the formation of the SNARE complex essential for neurotransmitter release. However, they target different neurons:
Tetanus toxin primarily affects inhibitory interneurons in the spinal cord, leading to muscle spasms and rigidity due to the inability to release inhibitory neurotransmitters.
Botulinum toxin targets peripheral motor neurons, inhibiting acetylcholine release at the neuromuscular junction, resulting in muscle paralysis.
How do tetanus and botulinum toxins differ in the SNARE proteins they target?
Tetanus toxin and BoNT B, D, F, and G all target and cleave VAMP (synaptobrevin), a SNARE protein essential for neurotransmitter release.
However, other botulinum toxin types (e.g BoNT/A & BoNT/E) can also cleave SNAP-25 and syntaxin.
What are some clinical uses of botulinum neurotoxins (BoNTs) beyond cosmetic applications?
strabismus (crossed eyes)
blepharospasm (uncontrollable blinking)
hemifacial spasm
cervical dystonia
axillary hyperhidrosis (excessive underarm sweating)
overactive bladder
gastrointestinal disorders
sialorrhea (excessive saliva)
temporomandibular disorder
limb spasticity.
Which type of botulinum toxin is most commonly used in therapeutic applications, and what is its target?
Botulinum toxin type A.
Targets SNAP-25.
This results in long-lasting effects, with treatments typically lasting for several months.
Why are medicines based on tetanus toxin not used clinically?
Because most individuals are vaccinated against tetanus, which would neutralize the therapeutic effects of the toxin.
Why are botulinum neurotoxins (BoNTs) widely used as therapeutic proteins?
High efficacy, long duration of action, tolerance, and favorable safety profile. This has made BoNTs among the most widely used therapeutic proteins globally.
What are the cosmetic and therapeutic uses of Allergan’s Botulinum A product?
Widely used for cosmetic purposes, such as reducing wrinkles and fine lines. It is also approved for various therapeutic indications, including treating strabismus, cervical dystonia, blepharospasm, overactive bladder, and limb spasticity.
How does botulinum toxin type A provide cosmetic benefits?
Temporarily relaxes facial muscles by blocking acetylcholine release at neuromuscular junctions.
Preventing muscle contraction, reducing the appearance of wrinkles and fine lines.
How can Botulinum toxin type A be used to treat strabismus nonsurgically?
Can be injected into the eye muscles to temporarily weaken overactive muscles responsible for strabismus (misaligned eyes). By relaxing the affected muscles, it helps align the eyes, providing a nonsurgical treatment option for this condition.
How is Botulinum toxin type B used to treat cervical dystonia?
Cervical dystonia = condition causing involuntary muscle contractions in the neck.
The toxin is injected into specific neck muscles to reduce muscle stiffness and abnormal head positioning, providing relief from pain and improving neck movement.
How does Botulinum toxin type B work to achieve chemodenervation?
Consists of a heavy chain and a light chain.
The heavy chain binds to synaptotagmin on neurons, allowing the toxin to be internalized into synaptic vesicles.
Once inside, the light chain cleaves synaptobrevin (VAMP), preventing acetylcholine-containing vesicles from fusing with the cell membrane.
This blocks acetylcholine release, leading to chemodenervation and temporary muscle relaxation.