IPS 1 -Phypharm [Rationale] Top Question with most Error
1/24
Earn XP
Description and Tags
Proverbs 16:3
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
D. 96.78 mL
48. Calculate the volume of NaCl solution to be added to satisfy this compounding.
Epinephrine (E = 0.29) - 0.5%
Sterile water, ad q.s. - ad 20 mL
Sodium chloride 0.9%
Make isotonic solution - 100 mL
A. 3.22 mL
B. 3.23 mL
C. 96.77 mL
D. 96.78 mL
A. Amount of drug removed
97. In zero order rate of reaction, which of the following is constant?
A. Amount of drug removed
B. Fraction of drug removed
C. Half-life
D. A and C
A. 0.45
60. Determine the sedimentation volume of a 2.5% w/v suspension of aspirin in water. The initial volume of the suspension was 100 mL while the volume of sediment was found to be 45 mL.
A. 0.45
B. 2.22
C. 0.45 mL
D. 2.22 mL
B. II and III
Sieve analysis:
I. Each aperture has square opening per cm inch
II. The finest particles pass through the highest sieve number
III. Official USP method
A. I and II
B. II and III
C. I and III
D. I, II and III**
D. Any proportion
Based on Figure 1, at what proportion will the system exist as one phase when the temperature is 80°C?
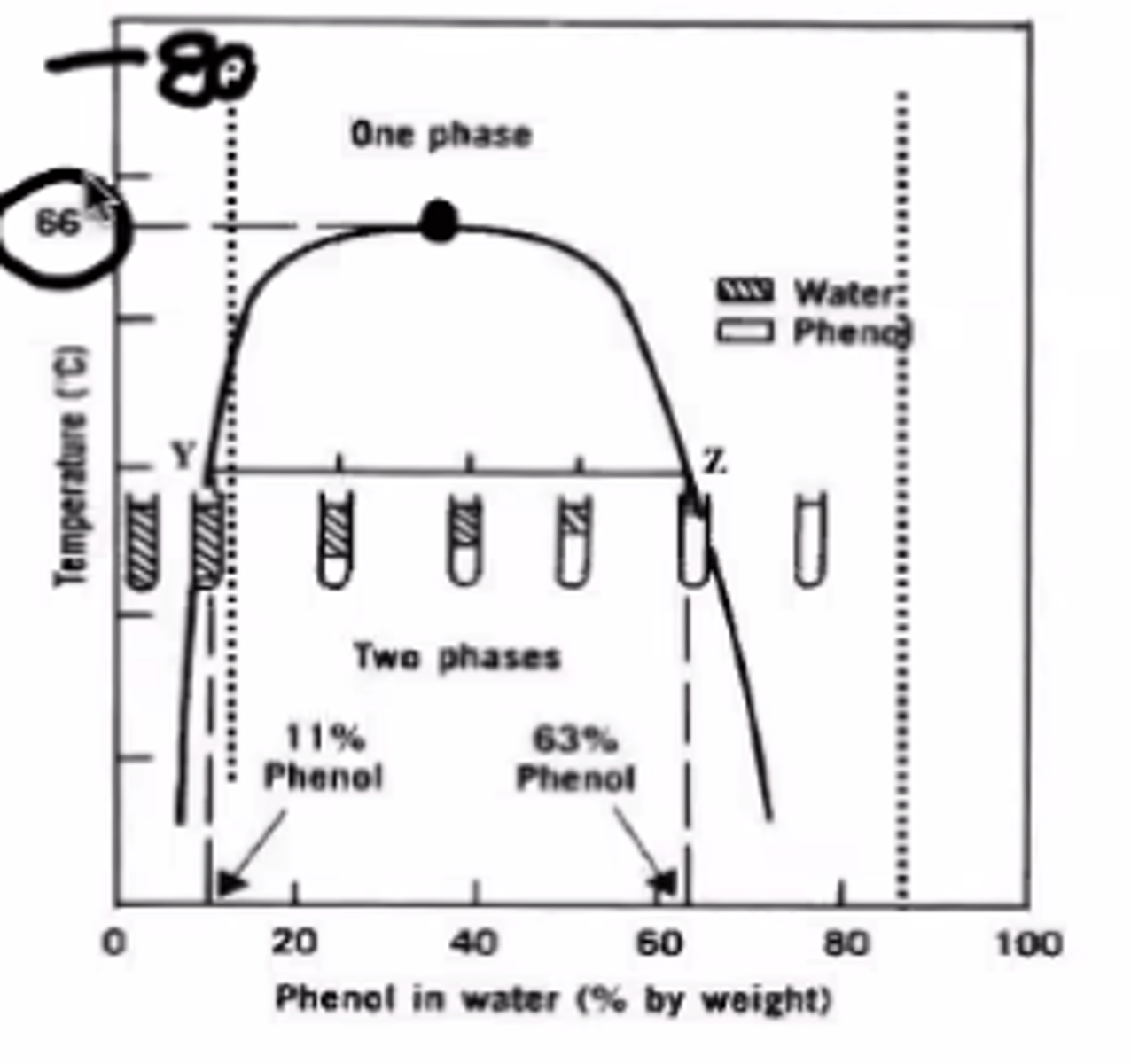
A. 80% only
B. 40% only
C. 10% only
D. Any proportion
E. Not achievable
D. A and B
The figure 1 illustrates what type of dispersion system?
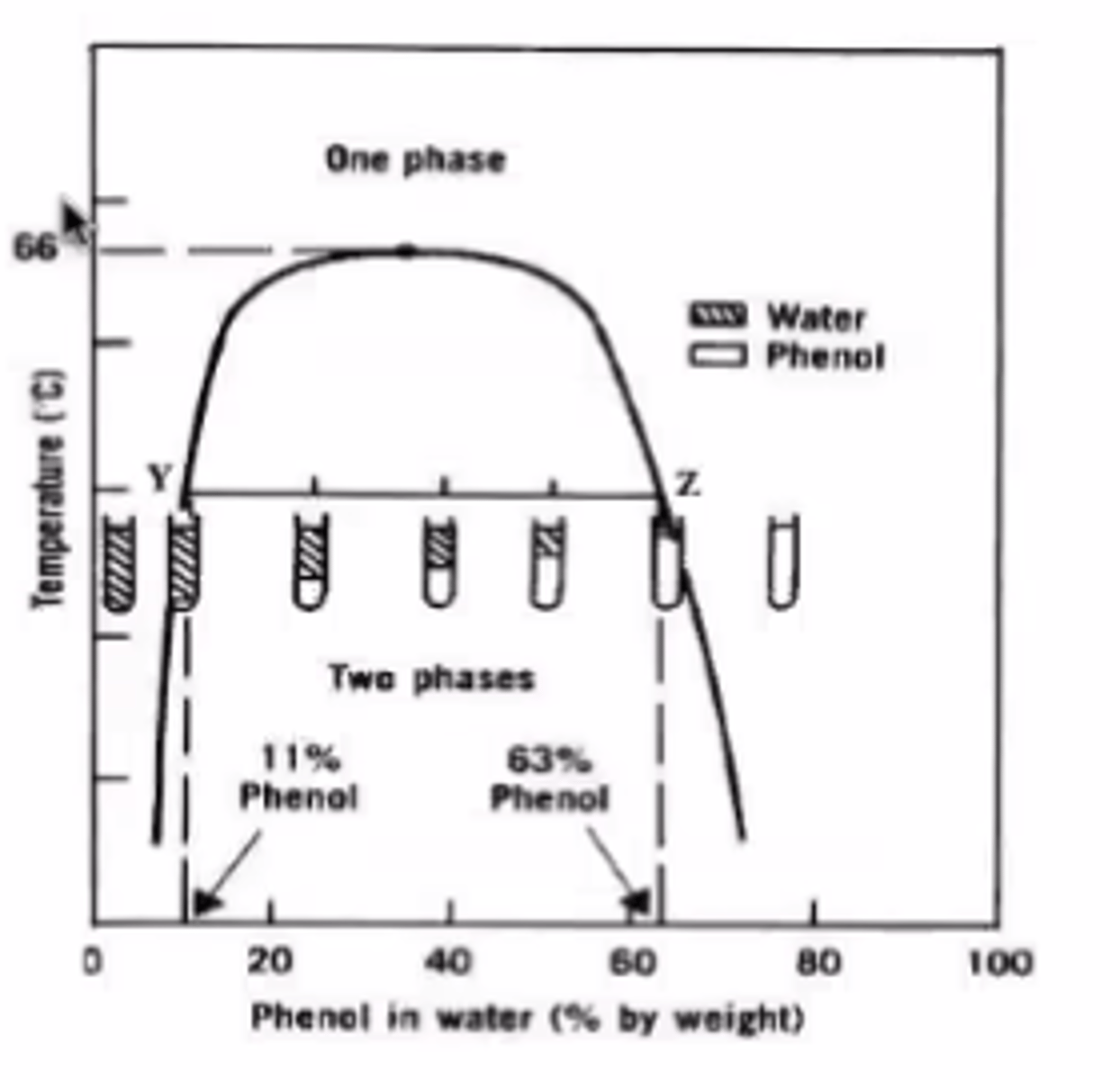
A. Condensed
B. Liquid-Liquid
C. Solid-Liquid
D. A and B
E. A and C
B. Rhombohedral packing
86. Which of the following has better flow?
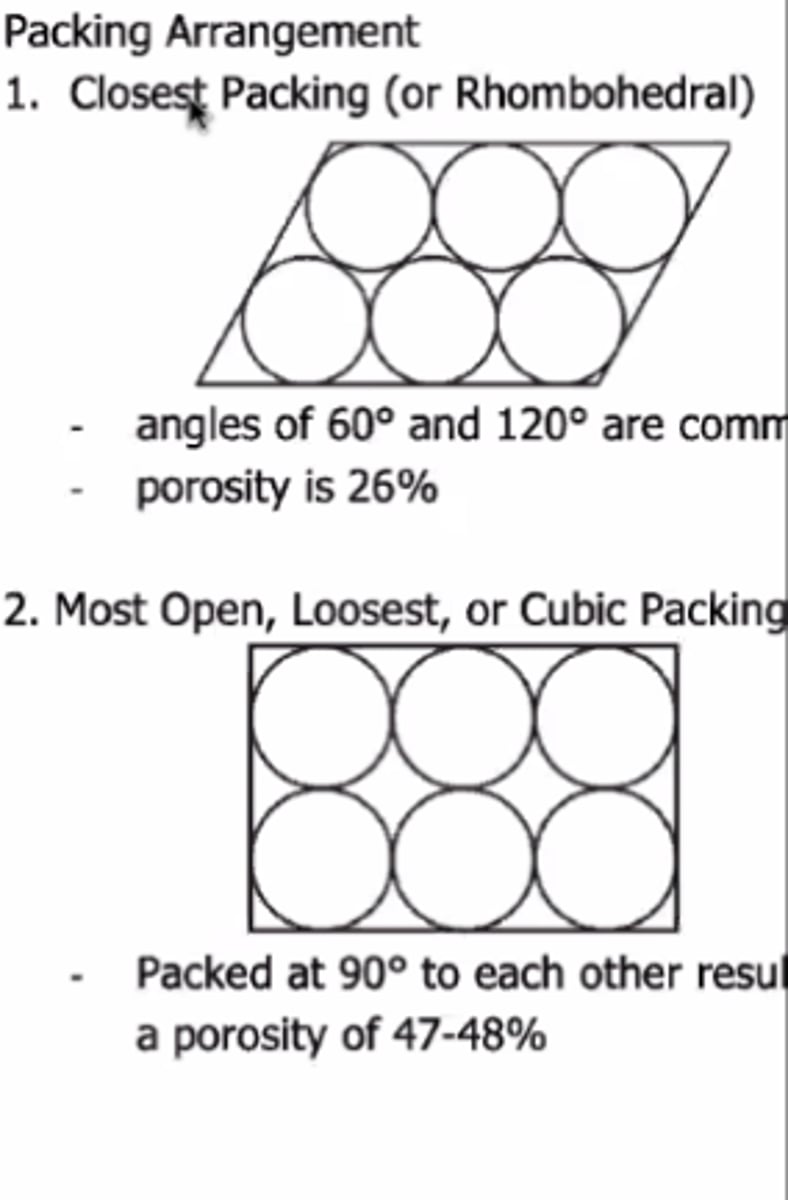
A. Loosest packing
B. Rhombohedral packing
C. Cubic packing
D. A and C
B. Moderately coarse
78. These particles are retained in sieve #60.
A. Very coarse
B. Moderately coarse
C. Fine
D. Coarse
A. Liquid crystalline
Mesophase:
A. Liquid crystalline
B. Intermediate between liquid and gas
C. Supercritical fluid
D. B and C
E. All
C. KCl
17. Which of the following will increase its solubility with increasing temperature?
A. Gas
B. Ca(OH)₂
C. KCl
D. A and B
(I) Feret's Diameter
Feret's Diameter:
• Distance between imaginary parallel lines tangent to a randomly oriented particle
• Lines are perpendicular to the ocular scale
Martin's Diameter:
• The diameter that divides a randomly oriented particle into two equal projected areas
Projected Area Diameter:
• Diameter of a circle having the same projected area as the particle
(I) Feret's Diameter
(II) Martin's Diameter
(III) Projected Area Diameter
B. 0.82 g
Calculate the quantity of boric acid needed to make the prescription below isotonic.
Atropine sulfate (E = 0.13) — 3%
Boric acid (E = 0.50) — q.s.
Sterile water for inj., ad — 80 mL
Sig: Make isotonic solution
A. 0.81 g
B. 0.82 g
C. 0.4 g
D. 0.41 g**
C. Water and ether
28. Which of the following liquid-liquid mixtures is the least characterized by complete miscibility?
A. Benzene and carbon
B. Alcohol and acetone tetrachloride
C. Water and ether
D. Glycerin and alcohol
A. Only 1st statement is true
10. Crystalline solids have sharp melting point. Amorphous solids are less soluble.
A. Only 1st statement is true
B. Only 2nd statement is true
C. Both true
D. Both false
D. I, II and III
Kinetic Molecular Theory for Gases
I. Individual molecules have different velocities
II. As the temperature lowers, the KE of gas molecules decreases
III. Particles exhibit continuous random motion
A. I only
B. II only
C. I and III
D. I, II and III
D. 0.74 g
46. How many grams of NaCl should be used in the compounding?
Ephredrine sulfate (E = 0.23) — 0.70 g
Sodium chloride — q.s.
Sterile water for inj., ad — 100 mL
Sig: Make isotonic solution
A. 0.16 g
B. 0.17 g
C. 0.73 g
D. 0.74 g
B. Flocculating agents
62. Neutral electrolytes that are capable of reducing the zeta potential of suspended charged particles to zero:
A. Dispersing agents
B. Flocculating agents
C. Suspending agents
D. Wetting agents
A. Henry's Law
24. The effect of pressure on the solubility of gases is expressed by this law which states that the concentration of dissolved gas is proportional to the partial pressure of the gas above the solution at equilibrium.
A. Henry's Law
B. Boyle's Law
C. Raoult's Law
D. Charle's Law
D. Coarse dispersion
27. Most pharmaceutical emulsions and suspensions are examples of _______.
A. Atomic dispersion
B. Molecular dispersion
C. Colloidal dispersion
D. Coarse dispersion
B. 4.46
41. What is the pH of a solution containing 0.10 moles of acetic acid and 0.05 moles of sodium acetate per liter of solution?
The pKa of acetic acid is 4.76.
A. 3.56
B. 4.46
C. 5.01
D. 5.06**
B. 10 to 30
16. A substance is considered to be soluble if one part of solute dissolves in ___ parts of solvent.
A. <1
B. 10 to 30
C. 100 to 1,000
D. >10,000
D. Gentisic acid
92. Caffeine forms a less soluble drug complex with which organic acid?
A. Benzoic acid
B. Salicylic acid
C. Caproic acid
D. Gentisic acid
A. Liquid to solid
3. What is the phase change involved when molar heat of fusion is released?
A. Liquid to solid
B. Solid to liquid
C. Gas to solid
D. Gas to liquid
A. Inorganic, chelates, olefin type, aromatic
94. Metal complexes include which of the following?
A. Inorganic, chelates, olefin type, aromatic
B. Inorganic, organic, quinhydrone type
C. Channel lattice, layer, clathrate
D. Inorganic, chelates, aromatic, clathrate
D. True density
84. Which of the following is determined by the He (helium) densitometer?
A. True volume
B. Bulk density
C. Granular density
D. True density