Shapes of simple molecules and ions
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Last updated 11:01 AM on 12/5/24
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
1
New cards
Linear
Cl-Be-Cl
2bp 0Lp
180
Equal
2
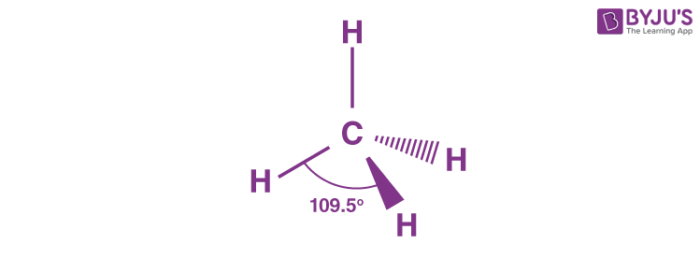
New cards
Tetrahedral
4bp 0Lp
109.5
Equal
3
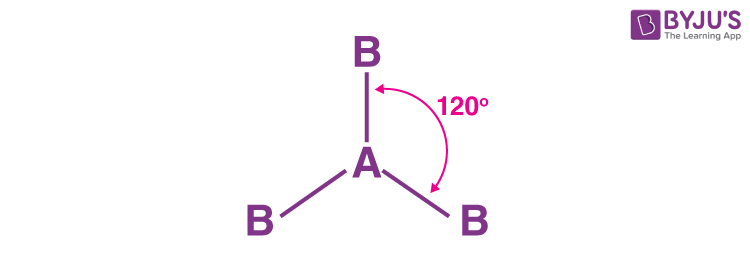
New cards
Trigonal Planar
3bp 0Lp
120
Equal
4
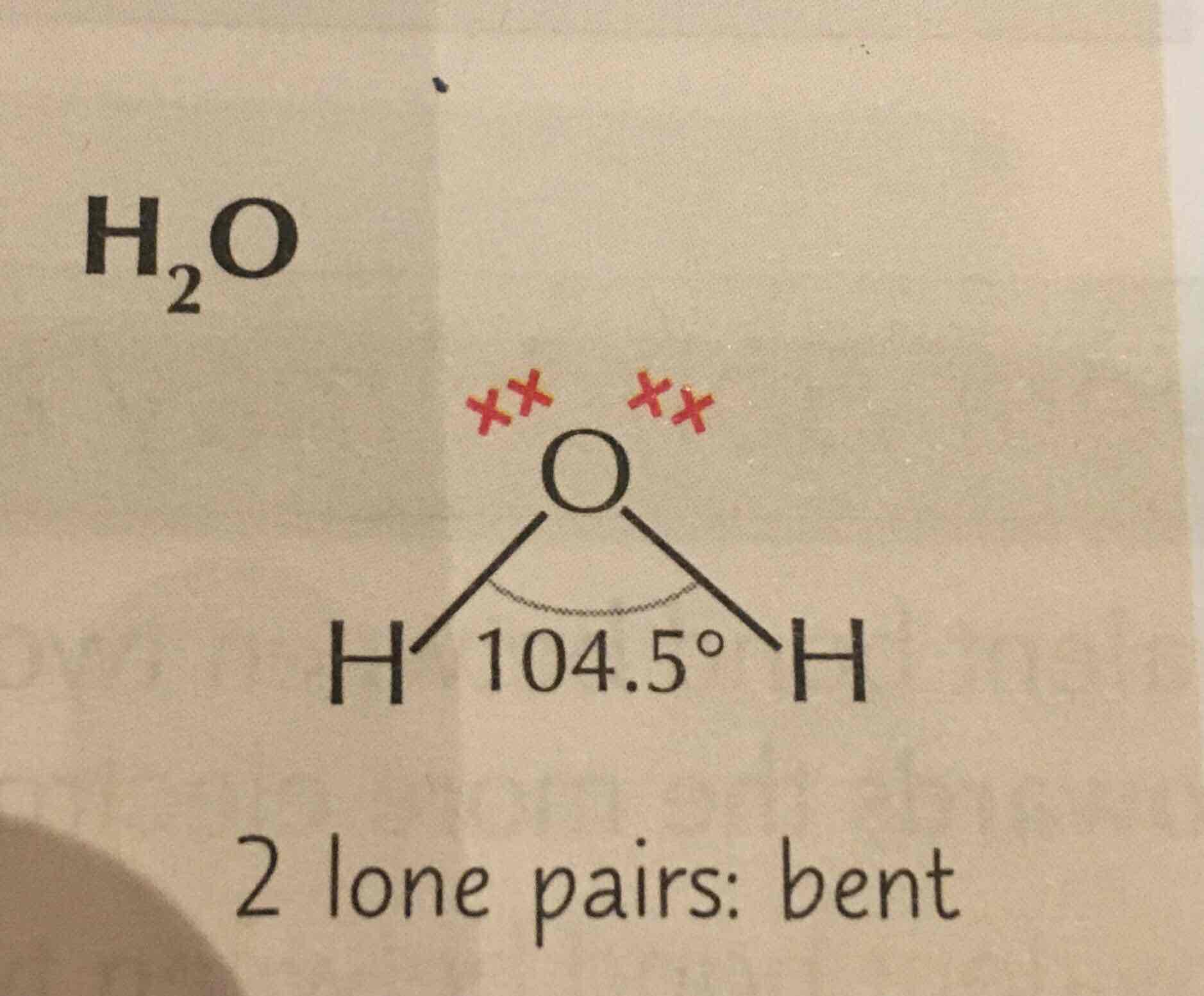
New cards
Bent/ non-linear/ v-shaped
2bp 2Lp
109.5-(2×2.5)=104.5
Unequal
5
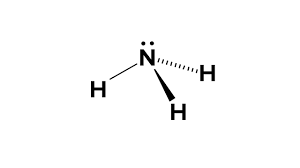
New cards
Trigonal pyramidal
3bp 1Lp
107
Unequal Ii
6
New cards
Trigonal bipyramidal
5bp 0Lp
Equal
120 and 90 y
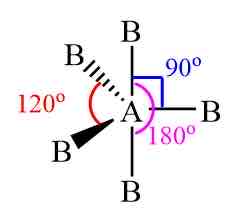
7
New cards
Octahedral
6bp 0Lp
Equal
90degrees
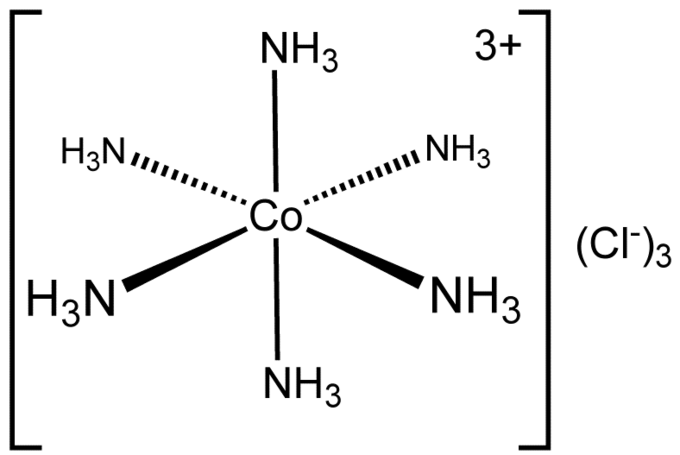
8
New cards
Square planar
4bp 2Lp
90degrees
Unequal
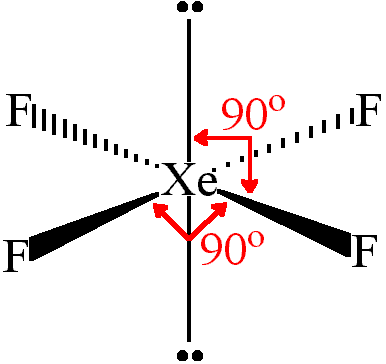
9
New cards
Explain the shape of molecules in words
State the number of bonding pairs and lone pairs(even if there is none)
State they are either repelling equally or not equally (lone pairs repel more than bonding pairs)
Gaining maximum separation with the bond angle ______