CHEM 1601 Exam 2 Memorize
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
alkane
C5 single bonds
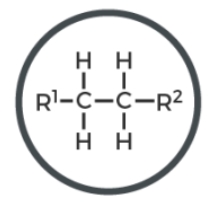
alkene
C double bond
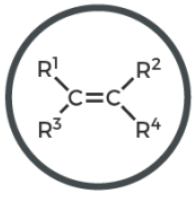
alkyne
C triple bond
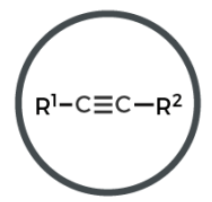
benzene
carbon ring w/ alternating single and double bonds
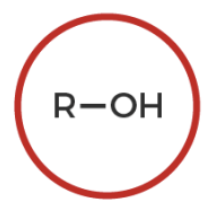
alcohol
R-OH
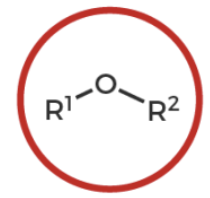
ether
R-O-R’
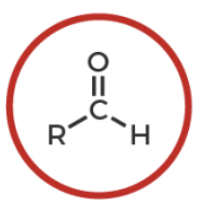
aldehyde
R-C(O double bond)-H
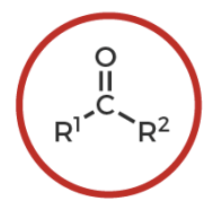
ketone
R-C(O double bond)-R’
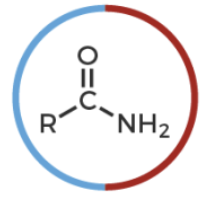
amide
R-C(O double nond)-NR’2
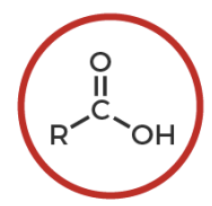
carboxylic acid
R-C(O double bond)-OH
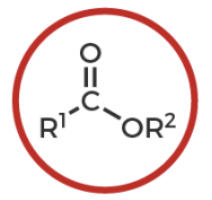
ester
R-C(O double bond)-O-R’
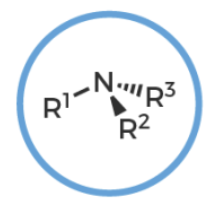
amine
R-NH2
nitrile
R-C(N triple bond)

linear electron geometry
2 electron domains
linear-linear
2 bonding 0 lone, 180º

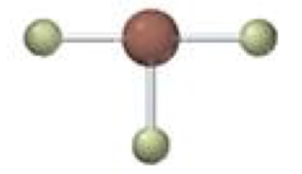
trigonal planar
3 electron domains
trigonal planar-trigonal planar
3 bonding 0 lone, 120º
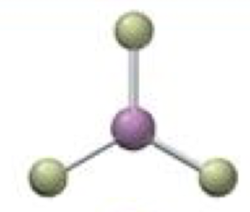
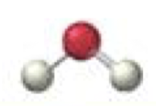
trigonal planar-bent
2 bonding 1 lone, <120º
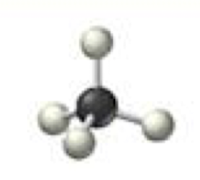
tetrahedral
4 electron domains
tetrahedral-tetrahedral
4 bonding 0 lone, 109.5º
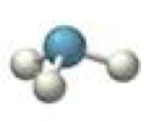
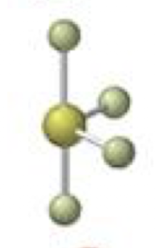
tetrahedral-trigonal pyramidal
3 bonding 1 lone, <109.5º
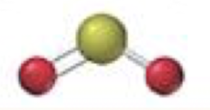
tetrahedral-bent
2 bonding 2 lone, <109.5º
trigonal bipyramidal
5 electron domains
trigonal bipyramidal-trigonal bipyramidal
5 bonding 0 lone, 120º equatorial 90º axial
trigonal bipyramidal-seesaw
4 bonding 1 lone, <120º equatorial <90º axial
trigonal bipyramidal-T-shaped
3 bonding 2 lone, <90º
trigonal bipyramidal-linear
2 bonding 3 lone, 180º

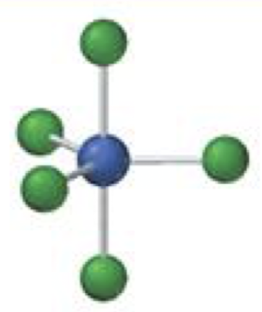
octahedral
6 electron domainso
octahedral-octahedral
6 bonding 0 lone, 90º
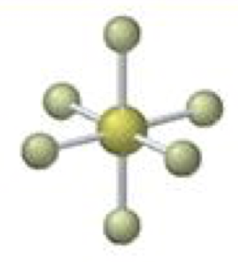
octahedral-square pyramidal
5 bonding 1 lone, <90º
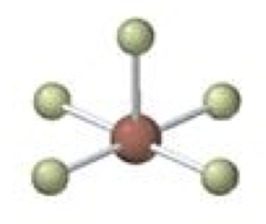
octahedral-square planar
4 bonding 2 lone, 90º
Cr e- config
[Ar]4s13d5 (half-filled d-orbital)
Mo e- config
[Kr]5s14d5 (half-filled d-orbital)
Cu e- config
[Ar]4s13d10 (full d-orbital)
Ag e- config
[Ar]5s14d10 (full d-orbital)
paramagnettic
UNPAIRED e-
diamagnetic
ALL PAIRED e-
H octet
2 e-
Be octet
4 e-
B octet
6 e-
Al octet
6 e-
H electronegativity
2.1
Cl electronegativity
3
S electronegativity
2.5
Period 2 electronegativity
F has 4, every element to left decreases by 0.5
nonpolar covalent bond electronegativity
∆0.0-0.5
polar covalent electronegativity
∆0.5-2.0
ionic bond electronegativity
∆>2.0
# of angular nodes
l
# of radial nodes
n-l-1
# of total nodes
n-1