Concentration of solutions.
1/3
Earn XP
Description and Tags
https://www.youtube.com/watch?v=3G3KQIyoZDI&list=PL9IouNCPbCxUhxxFUbR4SNfwmaRB8mYX3&index=16&ab_channel=Freesciencelessons
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
What is concentration
Concentration tells us the mass of a solute in a given volume of solution.
A solute is a chemical that is dissolved in a solvent
In chemistry, water is often used as a solvent
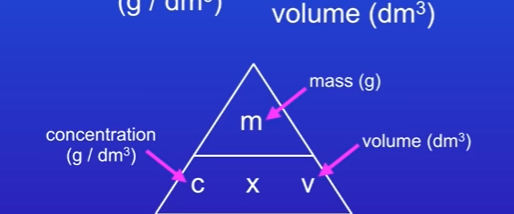
One unit of concentration is g/ dm³
How do we work out concentration
Concentration (g / dm³) = mass (g) / volume (dm³)
200g of chemical is dissolved in water to a final volume of 1 dm³. Calculate the concentration of the solution
Concentration = mass / volume.
200 / 1
= 200 g / dm³
How does mass of solute and volume of solution affect concentration.
If we increase the mass of solute and keep the volume the same, then we increase the concentration.
If we increase the volume of solution and keep the mass of the solute the same, then we decrease the concentration.