Nomenclature (Chemistry Terms)
1/103
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
104 Terms
Acetate
C₂H₃O₂⁻
Carbonate
CO₃²⁻
hydrogen carbonate (bicarbonate)
HCO₃⁻
Hydroxide
OH⁻
Nitrate
NO₃⁻
Nitrite
NO₂⁻
Chromate
CrO₄²⁻
Dichromate
Cr₂O₇²⁻
Phosphate
PO₄³⁻
Hydrogen Phosphate
HPO₄²⁻
Ammonium
NH₄⁺
Hypochlorite
ClO⁻
Chlorite
ClO₂⁻
Chlorate
ClO₃⁻
Perchlorate
ClO₄⁻
Permanganate
MnO₄⁻
Sulfate
SO₄²⁻
Sulfite
SO₃²⁻
hydrogen sulfite (bisulfite)
HSO₃⁻
hydrogen sulfate (bisulfate)
HSO₄⁻
Peroxide
O₂²⁻
Cyanide
CN⁻
Kilo
1000 - 10³ - thousand
centi
hundredth - 10-2 - 0.01
milli
thousandth - 0.001 - 10-3
deci
tenth - 0.1 - 101
How many inches in a foot
12 inches in 1 foot
What charge does Ag (Silver)
Plus 1 charge
What charge does Zn (Zinc) have
Plus 2 charge
Order of listing elements for molecular compounds
C, P, N, H, S, I, Br, Cl, O, F

Order of prefixes from 1 - 10
Mono, Di, Tri, Tetra, Penta, Hexa, Hepta, Octa, Nona, Deca
If an acid ends with “ite“ what should the ending be
-ous
if an acid ends with “ate“ what should the ending be
-ic
Ionic compounds are what with what
Metal and Nonmetal
Molecular compounds are what with what
Nonmetals only
Acids are what?
H and one or more nonmetals
Binary acids are what with what?
hydrogen and a nonmetal - HCL - Hydrochloric acid
Oxyacids are what with what
Oxygen with a hydrogen and a nonmetal
What is Cd (Cadmium) charge
+2
What is Ga (Galliums) charge
+3
What is In (Indium) charge
+3
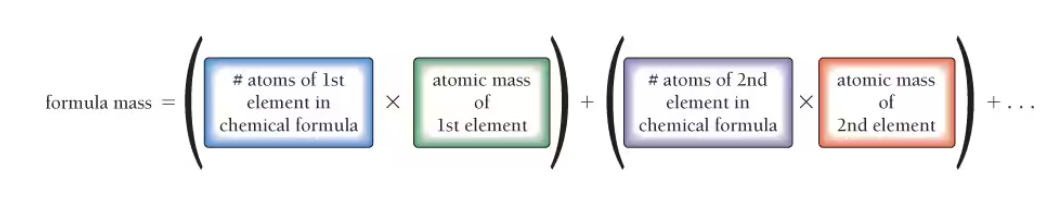
How to calculate the formula mass for both an element or a compound
Formula Mass for water is in Amu or g/mol and is the mols of an element times the Amu’s + the same thing for the other elements
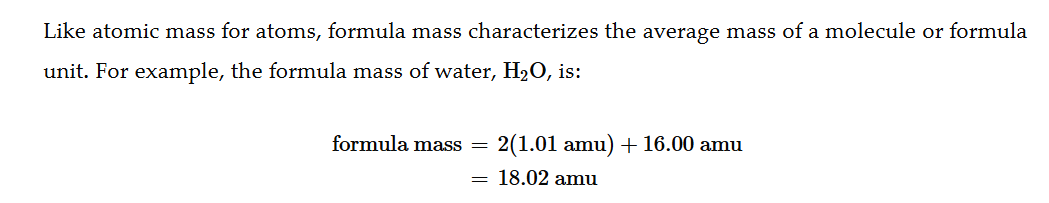
Avogadros Number
1 mol = 6.022 × 1023
Single element Mols to atoms and atoms to mols
Multiply mols by avogadros number (make sure to include units) to get atoms. Divide atoms by avogadros number to get mols
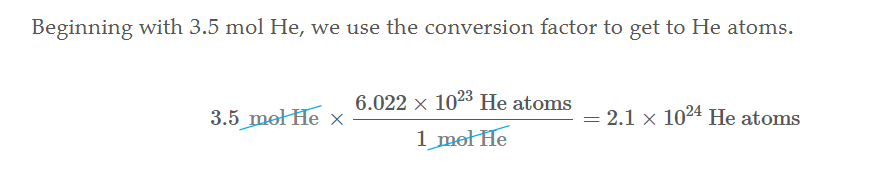
Single element Grams to mols
Divide the grams of the element by its amu to get mols. (Since amu can be written as mols per gram or grams per mol you can cancel them)
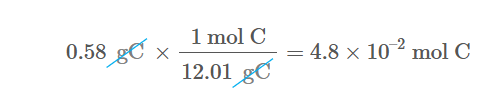
Single element Grams to atoms
Divide grams by amu, then multiply by avogadro’s number
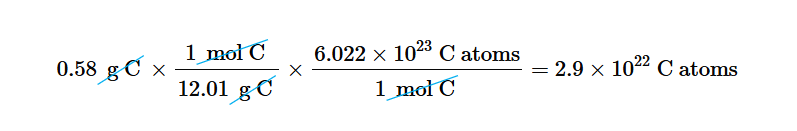
Compound Grams to mols
Get the formula mass (molar mass) of the whole compound. Then treat it like a regular single element equation.
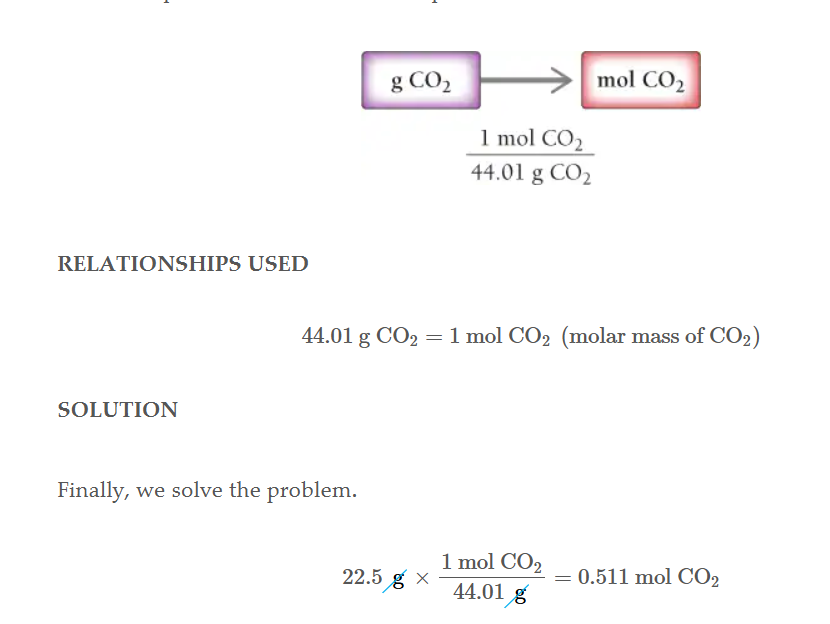
Compound grams to atoms.
Get the formula mass of the compound, change it to mols then multiply by avogadros number.
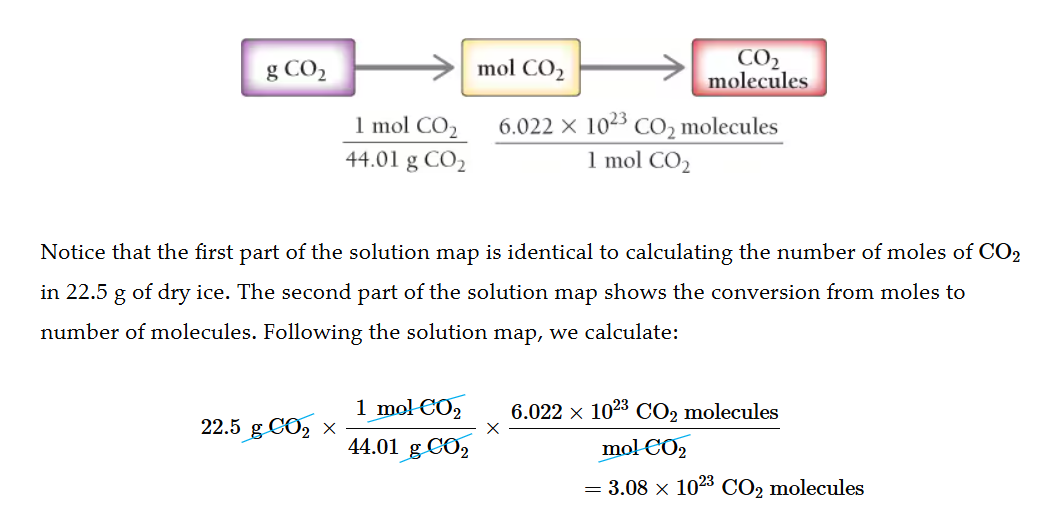
Number of mols of an element in a compound
Find the ratio (In H20 there are 2 H mols per 1 mol of H20) then cancel by the mols of H20 you have
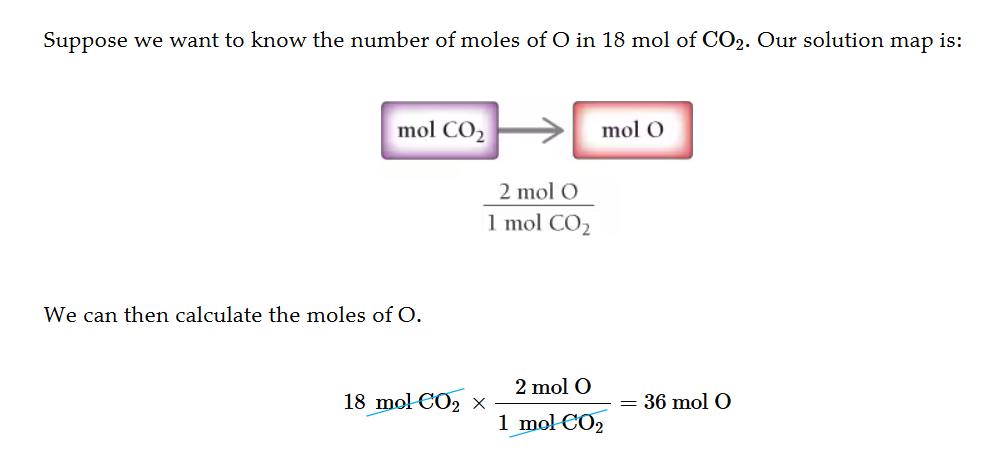
Finding the grams an element has in a compound
Change grams of the compound to mols of a compound, then find the ratio of mols of the element to mols of the compound, then convert the mols of the element to grams
Empirical formulas are what?
Compounds whos subscripts cannot be reduced (Such as H302C5, Unlike H202C2 which could be molecular)
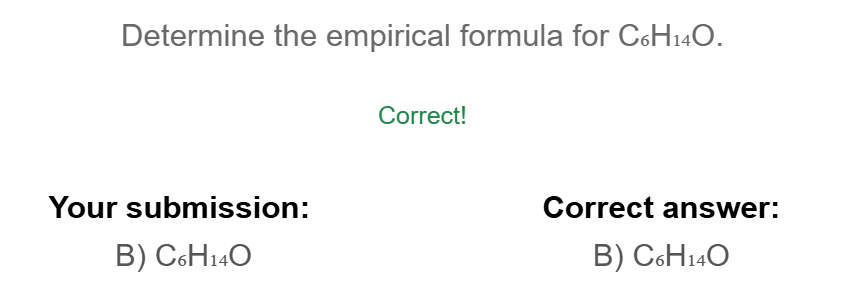
When using mass percents what should the bottom number be and what should the grams be
If you have 39.9% Cu then it should be 39.9 grams of Cu over 100 g of Cu02
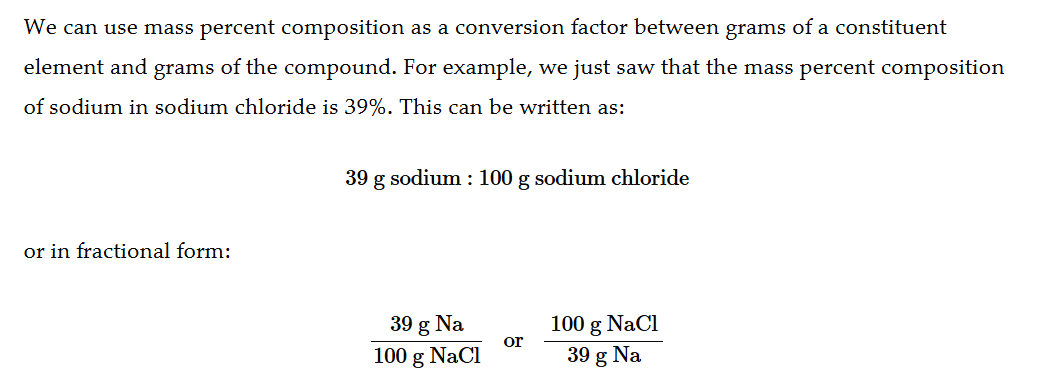
How to find mass percent for an element from grams of a compound
Divide the grams of the element you want to find by the grams of the compound you have, then multiply by 100% (move the decimal to the right)
How to find mass percent from a chemical formula
Divide the molar mass of the element by the molar mass of the compound (also multiply by the ration of the top to bottom)
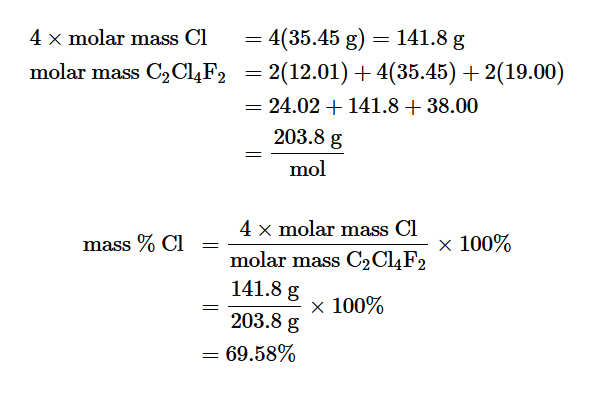
If the empirical formula numbers are not whole number what should you multiply with
These numbers (any just try to get the decimal to a whole number)
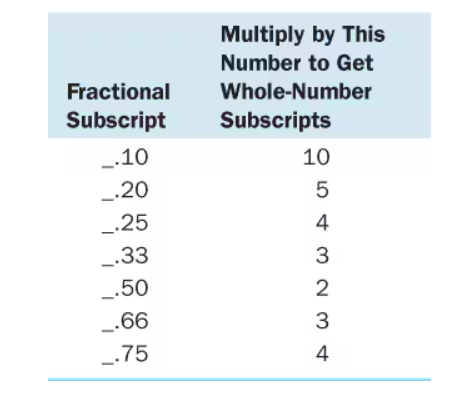
How to get empirical formula from grams
Change the grams to mols then shove them together, divide the mols of the compound by the mols of the smallest element, then multiply by the table of decimals to get a whole number (here it would be 2 resulting in N205, also try rounding to get 2 or 3 decimal places when getting the mols)
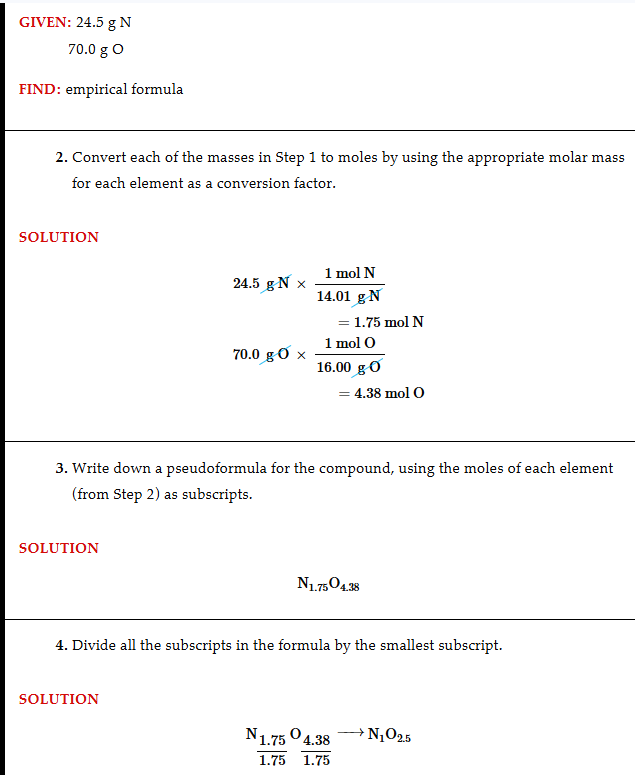
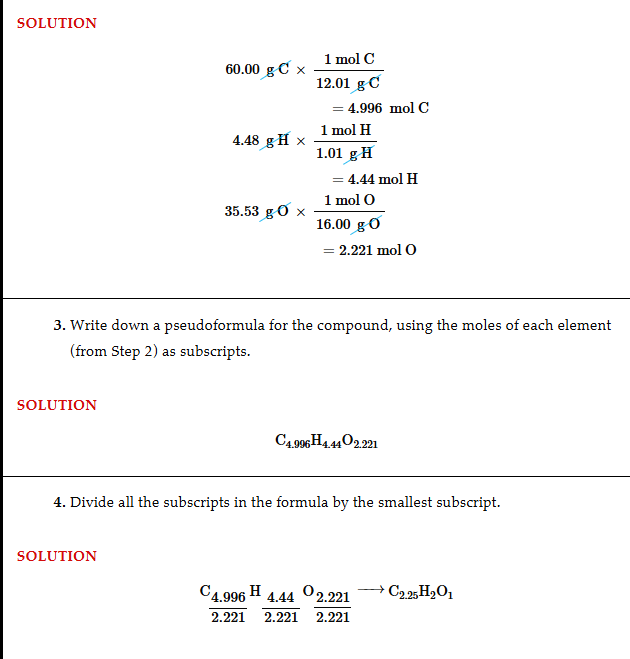
Getting empirical formula from percents
Change percents to grams, then those to mols, then divide by the smallest mol element, then multiply by the decimal table
Percent formulas add to what and how to get grams of an unknown element in a compound
Percent formulas add to 100% and if you have the grams of the compound and the grams of an element in the compound, conservation of mass lets you subtract the elements mass from the compound to get the rest.
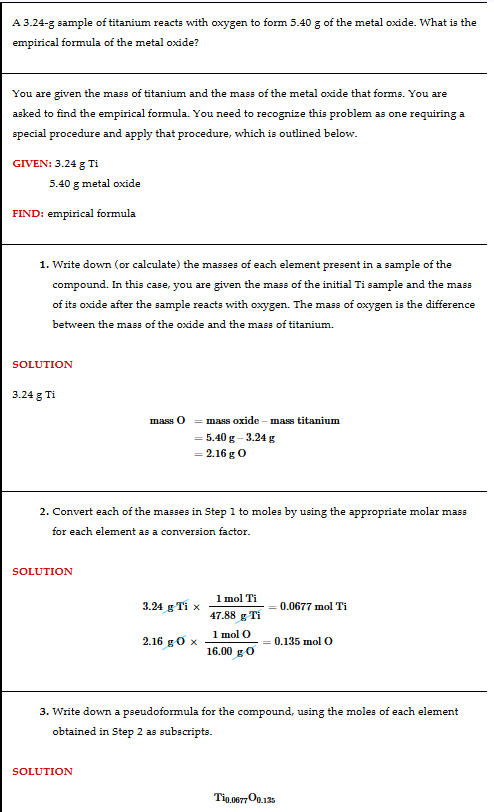
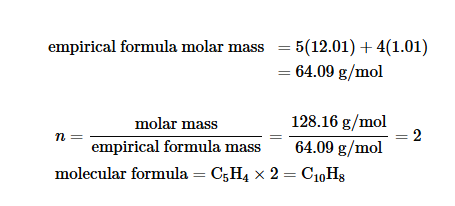
Getting molecular formulas from empirical formulas
You get the molar mass of the empirical formula and the molar mass of the molecular compound you want to find. You divide the molar mass of the unknown compound by the molar mass of the empirical formula to get a number. You multiply that number to the empirical formula to get a molecular formula.
Mega symbol, size is what?
M / 106
Kilo Symbol, Size is what?
k / 103 / 1000
Deci Symbol, Size is what?
d / 10-1 / 0.1
Centi Symbol, Size is what?
c / 10-2
Milli Symbol, Size is what?
m / 10-3
Micro Symbol, Size is what?
µ / 10-6
Nano Symbol, Size is what?
n / 10-9
1 lb = ? grams
453.59 grams = 1 ?
1 mile = ? km
1.609 km = 1 ?
1 cal = ? Joule
4.184 Joule = 1 ?
1 inch = ? cm
2.54 cm = ? inch
1 L = ? qt
1.0567 qt = ? L
1 L = ? mL
1000 mL = ? L
1 mL = ? cm3
1 cm3 = ? mL
1 atm = ? torr
760.00 torr = ? atm
°F = ?
(°C × 1.8) + 32
K = ?
°C + 273.15
density = ?
mass / volume
Mass Number
# of protons + # of neutrons
Atomic Number
# of Protons
Percent Natural Abundance
(Percent Natural Abundance of iso-1 / 100 Iso-1 Mass) + (PNA of iso-2 /100 * Iso-2 mass)….etc
Bromate
BrO3-
Hydrogen Sulfide
H2S-
Carbon, Nitrogen, Oxygen, Fluoride, Chlorine, Bromine, Iodine
When they have an O1 = They turn to -hypo (Hypochlorite - ClO-)
When they have an O2 = They turn to -ite (Carbonite - CO22-)
When they have an O3 = They turn to -ate
(Carbonate - CO32-)
When they have an O4 = They turn to -per
(Perbromate - BO4-)
Note: The charges also do not change
Phosphorous, Sulfur, Selenide, Arsenic
When they have an O2 = they turn into hypo-
(Hyposulfite - SO22-)
When they have an O3 = They turn to -ite
(Phosphite - PO33-)
When they have an O4 = They turn to -ate
(Selenate - SeO42-)
Note: The charges also do not change
Perflorate
FO4-
Perbromate
BrO4-
Periodate
IO4-
Selenate
SeO42-
Arsenate
AsO43-
Multiplying significant figures
The final answer will have the least number of significant figures.
3.95 × 0.2010 = 0.7935 is wrong (4 sig figs instead of 3)
0.794 is correct
Adding or subtracting significant figures
The final answer will have the least digits after the decimal
495+15.659+14.0 = 524.65 is wrong (2 decimal places instead of 0)
525 is right
How many sig figs are in 55.000
5 sig figs (any zero after a decimal is a sig fig)
How many sig figs are in 0.00600
3 sig figs (any zero before a nonzero is not a sig fig, but any number after is)
Hydronium
H3O+
When finding molar mass or moles, the sig figs to use are the ones from the first number
46.68 g N * 1 mol/14.007 = 3.333 (the starting number had 4 sig figs)
Diatomic Elements
H2, O2, N2, F2, Br2, I2
Strong Acids
HCl, HBr, HI |
HNO3 |
HClO4 |
H2SO4 |
Strong Bases
LiOH, NaOH, KOH |
Ca(OH)2 (slightly soluble) |
Ba(OH)2 |
Sr(OH)2 |
When you have strong bases or acids
separate bases and acids (H+ (aq) + Cl- (aq) )
Keep weak acids together (NH3)
Soluble Compounds (With Exceptions)
All sodium (Na+), potassium (K+), and ammonium (NH4+) salts are SOLUBLE
All nitrate (NO3-), acetate (CH3CO2-), chlorate (ClO3-), and perchlorate (ClO4-) salts are SOLUBLE
All chloride (and that means ANYTHING WITH Cl) (Cl-), bromide (Br-), and iodide (I-) salts are SOLUBLE — EXCEPT those containing: lead, silver, or mercury (I) (Pb2+, Ag+, Hg22+) which are NOT soluble.
All sulfate (SO42-) salts are SOLUBLE — EXCEPT those containing: calcium, silver, mercury (I), strontium, barium, or lead (Ca2+, Ag+, Hg22+, Sr2+, Ba2+, Pb2+) which are NOT soluble.