Lecture 2: vaccine immunology
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
describe how an infection in mice lacking innate vs adaptive vs normal mice would look
lacking innate: infection would increase exponentially as there is nothing to slow immediate infection, no APC to initiate adaptive
lacking adaptive: after initial plateau thanks to innate immune system interference, the levels of infection would slowly increase as there is no adaptive system to fully get rid of it
normal: initial spike, then plateau (innate IS), then decrease when adaptive response activated
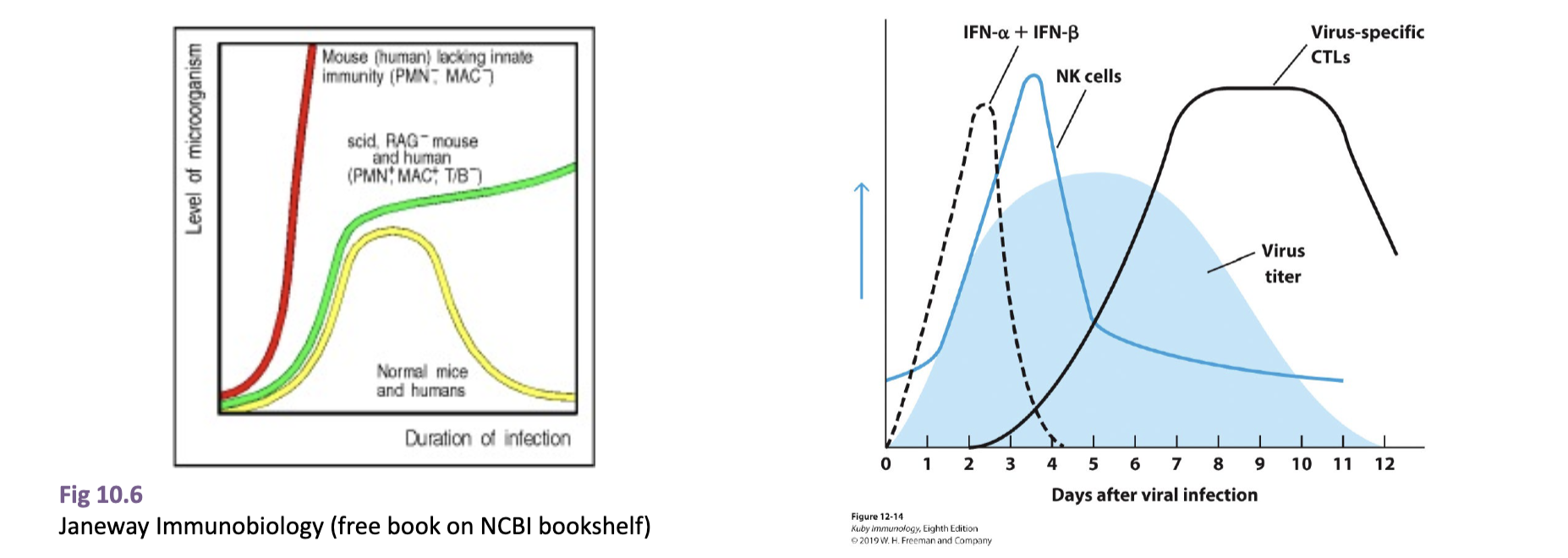
describe what occurs in the initial immune response at the site of infection
following breach, pathogen is attacked by resident cells at site of infection → APCs gather process the antigen for presentation. Activated DCs move via afferent lymphatics to draining lymph node to present antigen to adaptive cells
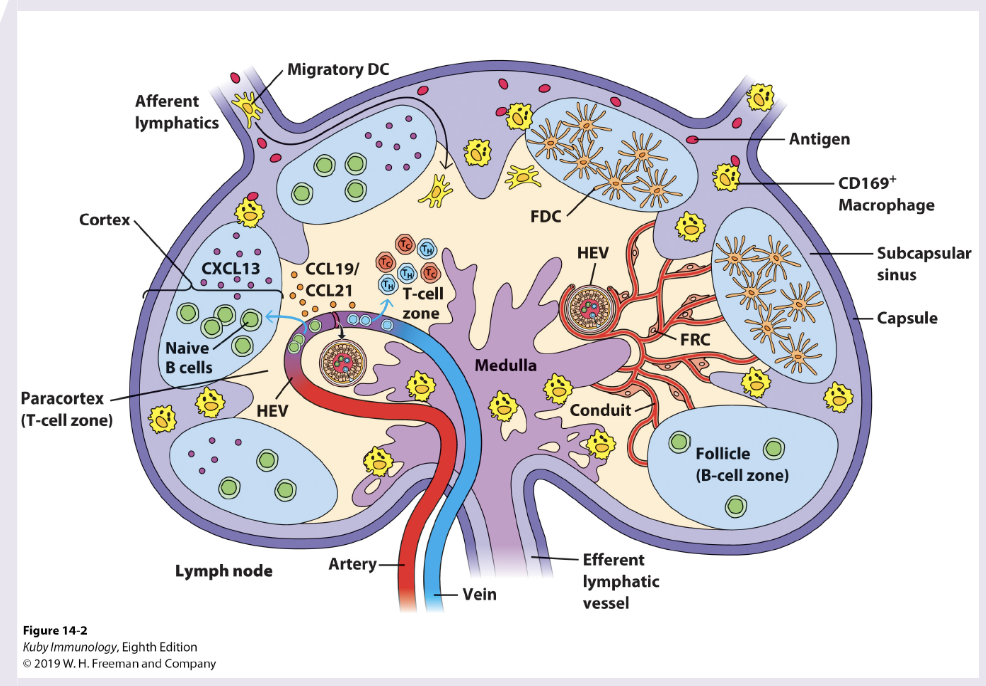
describe what happens in the different regions of the lymph node
T cell zone (Paracortex): DCs recruited by CCL19/21, naive CD8+/4+ T cells enter via HEVs, T cells scan DCs for cognate peptide-MHC complexes. Key outcomes: CD4 T cell priming & differentiate into TH1,2,17, fh, CD8+ priming (diff into CTLs), activated T cells undergo clonal expansion
B cell Follicles (Cortex): naive B cells reside and encounter native antigen delivered via lymph or macrophages. Ag binds BCR, providing first signal for B cell activation. Key outcomes: activated B cells upregulate CCR7 and migrate to border between B and T cell zone
T-B cell border: Ag-specific B cells present peptide-MHC II to activated Tfh cells. CD40/CD40L interactions and cytokines provide T cell help. key outcomes: B cells commit to either extrafollicular responses or germinal centre entry
extrafollicular response (outer cortex/medullary cords): rapid diff of B cells into short-lived plasmablasts, early secretion of low-affinity IgM to provide rapid protection in acute infection
Germinal centre (within B cell follicle): formation of GC with distinct zones: dark zone B cell proliferation and somatic hypermutation, light zone selection by follicular DC and Tfh cells. key outcomes: affinity maturation, class switching, generate plasma cells, memory B cells
what are the different broad types of immune responses against infection?
Type 1 response: TH1 driven, vesicular or cytosolic, CTL2, cytokines, NK cells
Type 2 respons: Th2 driven, extracellular
Type 3 response: TH17 driven, extracellular
describe the humoral response to viruses
B cells
secretory IgA → block binding of virus to host cell
IgG, IgM, IgA → block fusion of viral envelope to host cell p memb
IgG and IgM → enhance phagocytosis of viral particles (opsonization)
IgM → agglutinates viral particles
complement activated by IgG or IgM → mediates opsonizatio. by C3b and lysis of enveloped viral particles by membrane attack complex
describe the cell mediated immune response to viruses
IFN-g secreted by Th or Tc cells → has direct antiviral activity
cytotoxic T lymphocytes → kill virus-infected cells
NK cells and macrophages → kill virus-infected cells by antibody-dependent cell-mediated cytotoxicity
what are the key steps to antigen presentation?
Step 1: acquisition of antigens
MHCI – cytosolic Ags
MHCII – extracellular Ags acquired through phagocytosis and endocytosis
Cross-presentation and autophagy
Step 2: tagging antigens for proteolysis into peptides
Step 3: proteolysis
Step 4: delivery of antigens to MHC molecules
Step 5: loading of peptides onto MHC molecules
Step 6: display of MHC molecules at the cell surface
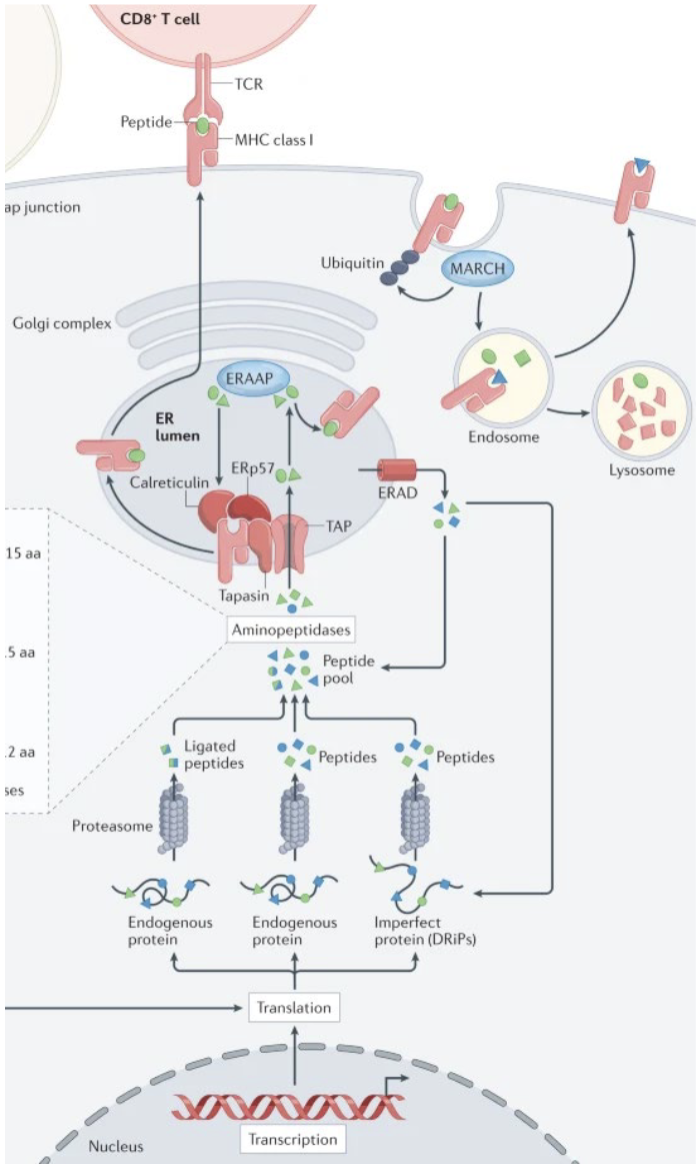
describe how antigens are presented on MHC class I
Generation of endogenous antigens from cytosolic proteins (defective ribosomal products, DRiPs) (e.g., viral, tumor, or self proteins)
Proteasomal degradation of proteins into peptide fragments
Transport of peptides into the ER via TAP1/TAP2 transporters
Synthesis and folding of MHC class I heavy chain in the ER
Association with β2-microglobulin (β2m) to form the MHC I heterodimer
Stabilization of MHC I by chaperones (calnexin, calreticulin, ERp57)
Peptide loading onto MHC I facilitated by the peptide-loading complex (including tapasin)
Selection of high-affinity peptides, stabilizing the MHC I complex
Transport of peptide–MHC I complexes through the Golgi to the cell surface
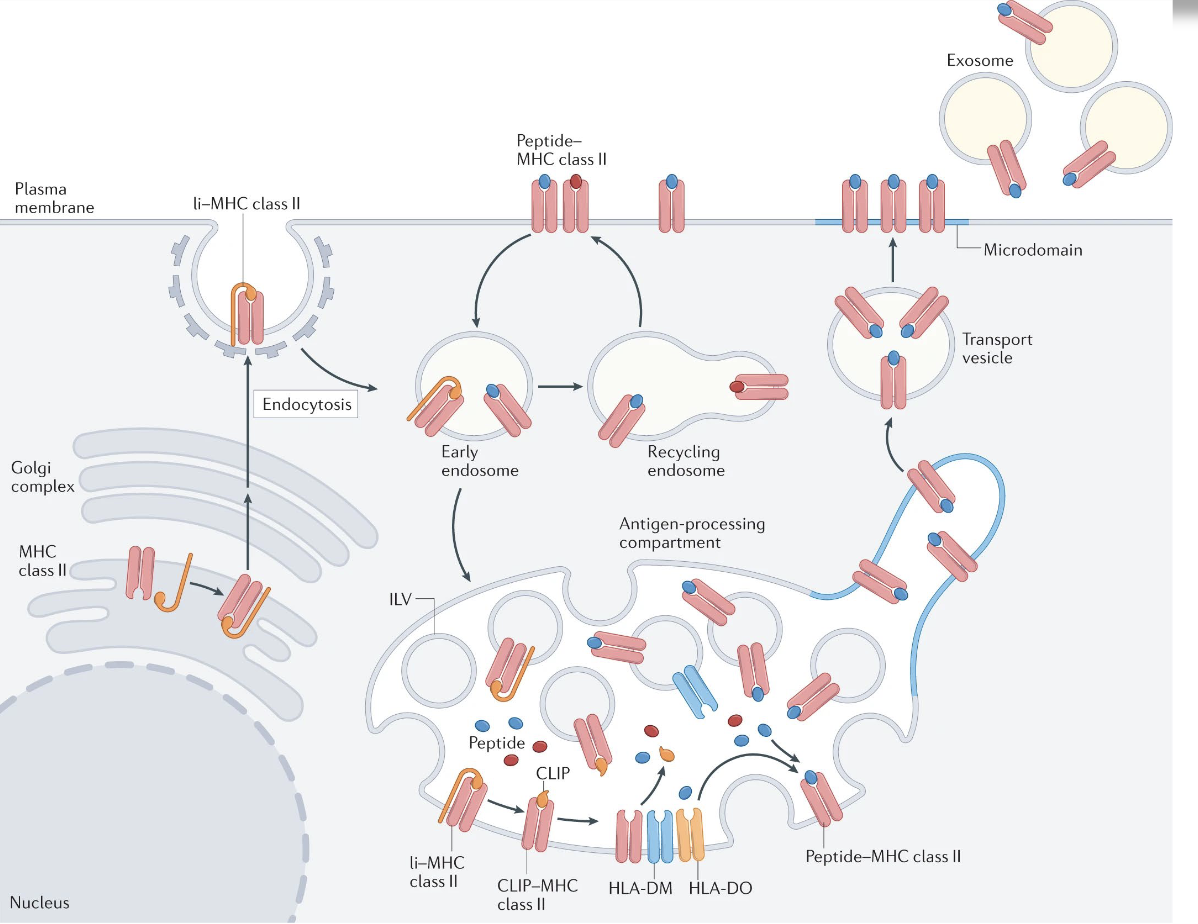
describe how antigens are presented on MHC class II
Antigen uptake by professional APCs (dendritic cells, macrophages, B cells) via endocytosis or phagocytosis
Proteolytic processing of antigen in acidified endosomes/lysosomes
Synthesis of MHC class II α and β chains in the endoplasmic reticulum (ER)
Association with invariant chain (Ii/CD74) in the ER to block the peptide-binding groove
Trafficking of MHC II–Ii complexes from the ER through the Golgi to late endosomal compartments (MIIC)
Proteolytic degradation of invariant chain, leaving CLIP in the peptide-binding groove
Exchange of CLIP for antigenic peptide mediated by HLA-DM (modulated by HLA-DO in some APCs)
Stabilization of peptide–MHC II complexes
Transport of peptide–MHC II to the cell surface
can CD8+ T cell responses be generated against extracellular antigens?
yes, through cross presentation
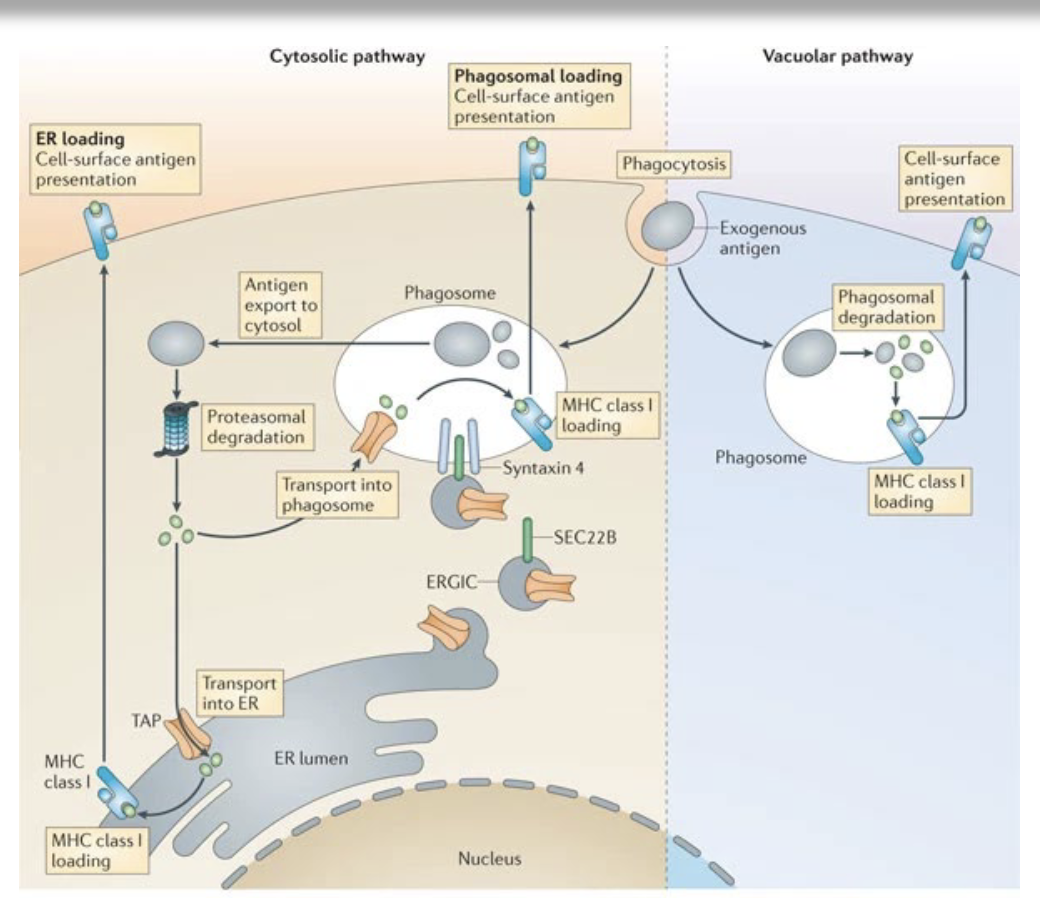
describe the process of cross-presentation
Uptake of exogenous antigen by specialized antigen-presenting cells (primarily dendritic cells) via phagocytosis or endocytosis
Antigen retention in early endosomes or phagosomes with limited acidification to prevent excessive degradation
Translocation of antigen from endosomes/phagosomes to the cytosol (cytosolic pathway) or processing within endosomal compartments (vacuolar pathway)
Proteasomal degradation of cytosolic antigen into peptides (cytosolic pathway)
Transport of peptides into the ER or ER–phagosome via TAP transporters
Loading of peptides onto MHC class I molecules with the help of the peptide-loading complex (e.g., tapasin)
Alternative vacuolar loading of peptides directly onto MHC class I within endosomes (TAP-independent pathway)
Stabilization of peptide–MHC class I complexes
Transport of peptide–MHC class I complexes to the cell surface
allows cells to present on MHC I without actually being infected
what happens during vaccination following antigen presentation?
activation of B/T cells in lymph nodes or secondary lymphoid tissue
APCs present antigen on either MHC class I, inducing activation of CD8+ T cells, or II, inducing CD4+ T cell activation which provides T cell help to B cells and CD8+
what types of T memory cells are found at which locations?
CD8+ and CD4+ memory T cells have tissue-specific localization and migration capacity
Central memory T cell (TCM): in humans are usually CD4+, circiulate and are found mainly in lumphoid tissue
effector memory (TEM): both CD4/8+ are found in the circulation and in lymphoid and mucosal tissues
tissue-resident memory T (TRM) cells localize mostly to mucosal tissues (lungs and intestines) and barrier sites and are also found in lymphoid organs.
note: bone marrow can also be a reservoir for memory cells
!!!describe how the immune response is initiated at different sites
at mucosal sites (eg lung), DC take up virus and present viral Ag in MHC, which then travel to draining lymph node to present to TCR of niave T cells, which differentiate into effector T cells and proliferate and circulate
describe what happens to B cells during infection
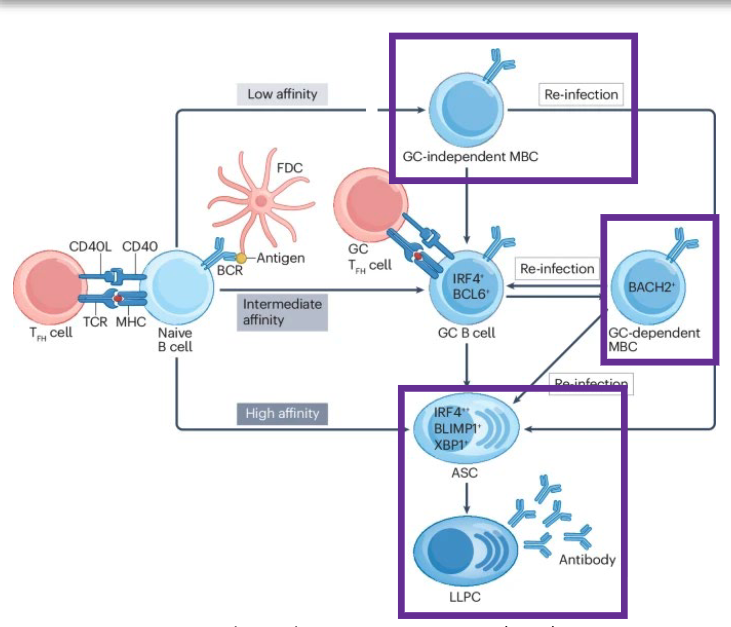
Naive B cells in the lymph node interact with CD4+ T fh cells to generate extrafollicular antibody-secreting cells (ASCs), germinal centre (GC)-independent memory B cells (MBCs) and GC B cells. B cells that enter the GC undergo somatic hypermutation and selection for affinity maturation of the B cell receptor (BCR) and can differentiate into MBCs or ASCs. ASCs (both extrafollicular and GC dependent) circulate until they arrive in tissue niches, wherein they can become long-lived plasma cells (LLPCs).
where can the types of memory B cells be found?
Both MBCs and ASCs can be found in circulation, whereas tissue-resident memory B (BRM) cells can be found in lymphoid and mucosal tissues, and LLPCs are mostly found in bone marrow and intestines.
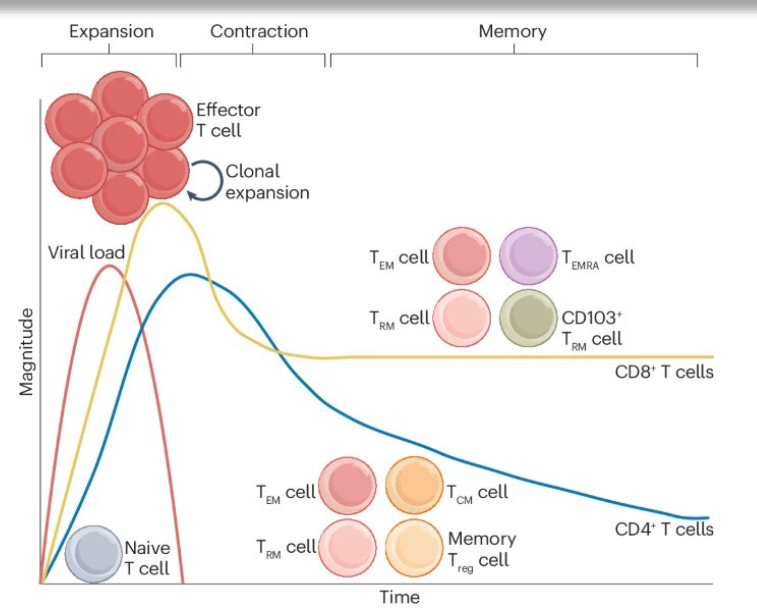
describe the phases of the T cell response following viral infection
expansion, contraction and memory phases. During the expansion phase, Ag-specific naive T cells differentiate into effector T cells, which rapidly undergo clonal expansion and secrete effector molecules such as pro-inflammatory cytokines and cytolytic effectors. CD8+ T cell populations expand to a greater magnitude than CD4+ T cell populations. Once the virus is cleared, contraction occurs, whereby most of the effector T cells undergo apoptosis. The remaining cells are then maintained as diverse memory T cell subsets for long time periods. TCM cell, central memory T cell; TEM cell, effector memory T cell; TEMRA cell, effector memory T cell re-expressing CD45RA; Treg cell, regulatory T cell; TRM cell, resident memory T cell.
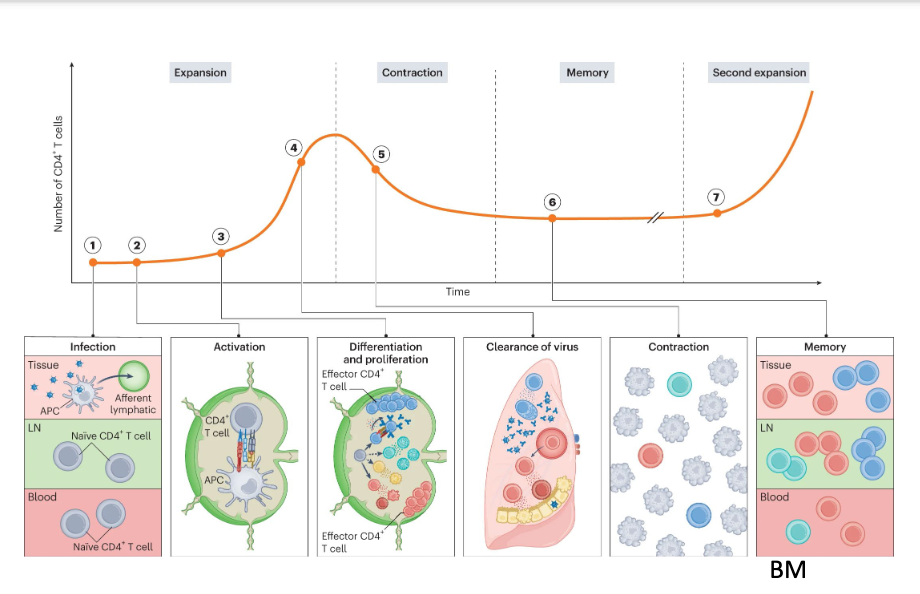
describe what happens to T cells over the course of an infection
1. Naive CD4+ T cells quiescently recirculate through blood (dark red) and lymphoid (light green) tissues. Upon infection, APCs migrate from infected barrier sites to the draining lymph nodes through afferent lymphatics and present peptides on MHC-II molecules. 2. Recognition of the peptide- MHC-II complex through TCR in combination with co-stimulation and cytokine signals lead to the activation, differentiation and expansion of naive CD4+ lymph node T cells. 3. CD4+ T cells proliferate and differentiate into various effector subsets that each become poised to make specialized contributions to immunity. 4. Many proliferated T cells leave the lymph nodes and migrate to the infected tissue through blood to assist in pathogen control at sites of infection. 5. Once the infection is cleared, most pathogen-specific CD4+ T cells die resulting in contraction of the population. 6. However, a few survive to establish long-lived memory and stay widely distributed across the body. 7. Upon reinfection, memory CD4+ T cells can mount anamnestic responses that are quicker and of higher magnitude than a primary
describe how B cells encounter Ag and collaborate with T cells
Naïve B cells bind antigen via the BCR in the follicle.
Antigen is internalized and presented on MHC class II.
Activated B cells migrate to the T-B border and receive help from cognate CD4* T follicular helper (Tfh) cells.
Key signals:
CD40-CD40L
Cytokines (e.g., IL-21, IL-4)
These signals license B cells to enter the germinal center program.
once in germinal centre, describe what happens in the dark zone vs light zone
2. Dark zone: clonal expansion and somatic hypermutation B cells (now called centroblasts) undergo rapid proliferation. Activation-induced cytidine deaminase (AID) introduces mutations into the immunoglobulin V regions (somatic hypermutation, SHM). Result: a diverse pool of BCR variants with different antigen affinities. No selection occurs here-only diversification.
3. Light zone: affinity-based selection. Mutated B cells (now centrocytes) migrate to the light zone. They compete for antigen displayed on follicular dendritic cells (FDCs). B cells with higher-affinity BCRs: Capture more Ag, Present more peptide-MHC II, Receive stronger survival signals from Th cells Low-affinity or autoreactive B cells undergo apoptosis.
4. Recycling between zones
Positively selected B cells can:
Re-enter the dark zone for additional rounds of mutation and selection (cyclic re-entry), or Exit the GC to differentiate This iterative process drives affinity maturation.
B cell class-switch recombination (CSR)
Also mediated by AID.
Guided by Tfh-derived cytokines:
IL-4 → IgG1/ IgE
IFN-y → IgG2a (mouse)
TGF-ß → IgA
Changes antibody effector function without altering specificity.
describe how antigen specificity of the BCR is determined
• Antigen specificity is determined by three hypervariable loops, known as complementarity-determining regions (CDR1, CDR2, CDR3), which form a surface complementary to the antigen.
form Ag-binding regions
have most mutations in CDR1, 2, 3
• Both heavy (VH) and light (VL) chain CDRs contribute to forming the antigen-binding site.
• Antigen binding depends on the specific combination of the heavy and light chains working together.
How to B cells and Tfh interact?
Tfh interact with cognate B cells:
• TCR engages peptide:MHC → B cells present Ag, Tfh interacts with TCR, provides signals
• Costimulation – CD40L engages CD40 → increases B cell activation
• Provision of soluble factors
describe TFH differentiation and how they are important for B cells
TH cells are classified by TFs and cytokines → TFH express STAT3, Bcl-6. B+Then, depending on which TFs they express, further differentiate infto TFH subsets → TFH1, TFH2, TFH3, which secreted different cytokines and induce class switching
TH1 → IFN-g IgG
TH2 → IgE
TFH17 → IgA
when does class-switching happen?
happens pre-GC
switched isotypes are detectable before morphologically-defined GCs
GC-deficient models (eg. Bcl6-/-) can still undergo CSR
Early extrafollicular plasmablasts are frequently class-switched but unmutated
what impact did T cell help have in the paper discussed
availability of T cell help available for antigen determined the Ab response
immune bias
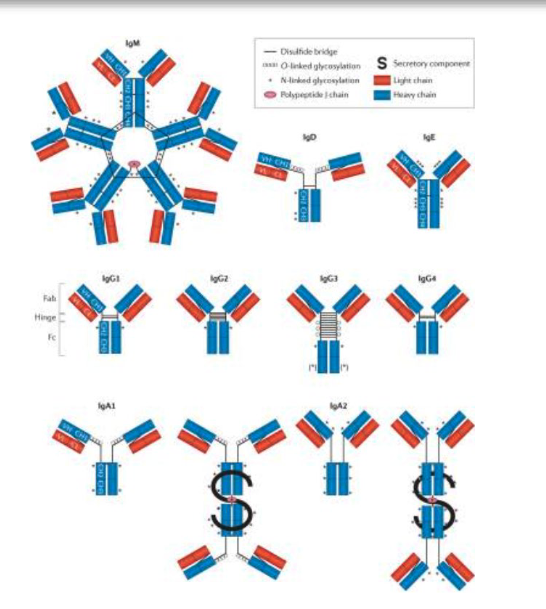
describe the structure of antibodies
two fxnl domains linked by a hinge region. The domains include an Ag-binding fragment (Fab) domain that binds to Ag and a crystallizable fragment (Fc) domain that binds to host sensors that deploy effector functions.
Ab molecule is composed of four chains with two identical heavy chains (blue) and two identical light chains (red). These are further divided into variable (VH or VL) domains and constant (CH or CL) domains, which form the Fab and the Fc domains. Fc domain diversity is generated during an immune response via the selection of different antibody isotypes, subclasses and post-translational glycosylation profiles.
linked by disulfide bridges
5 istoypes: IgM, IgD, IgG, IgA, IgE
IgA can exits as monomer in circulation, but dimeric (liked by J chain) at mucosal surfaces
IgM is pentamer
how does T cell affinity impact naive B cells?
affinity of Tfh cell binding (TCR to MHC II, CD40L to CD40) to naive B cell determines B cell differentiation:
low affinity: B cell becomes GC-independent memory B cell (MBC), whichh upon reinfection can reactivate
intermediate affinity: B cell becomes GC B cell, and eventually differentiates into GC-dependent MBC
High affinity: becomes ASC, which becomes long lived plasma cells (LLPC)
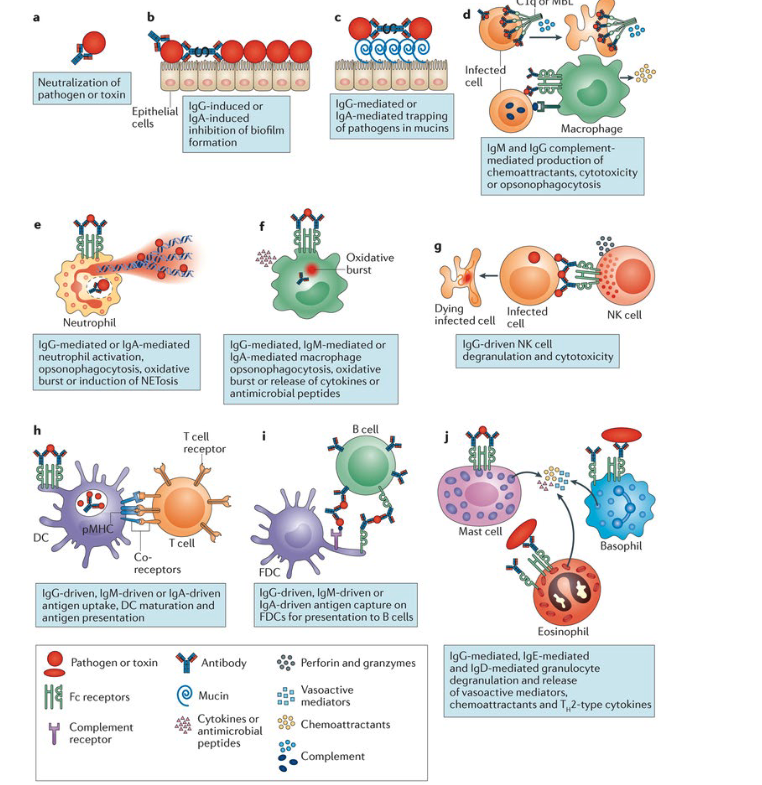
describe antibody functions in an infection setting
Neutralization of toxins and pathogens
Neutralization of microbial virulence factors → IgG and IgA inhibit biofilm formation
Pathogen trapping in mucus (IgG or IgA)
Complement activation and lysis → IgM and IgG → opsonizatoin
Neutrophil activation and NET formation
Macrophage phagocytosis and killing
NK cell–mediated cytotoxicity (ADCC)
Enhanced antigen presentation by dendritic cells
Antigen presentation by follicular dendritic cells
Mast cell, basophil, and eosinophil degranulation
NOTE: different anibody isotypes have different functions
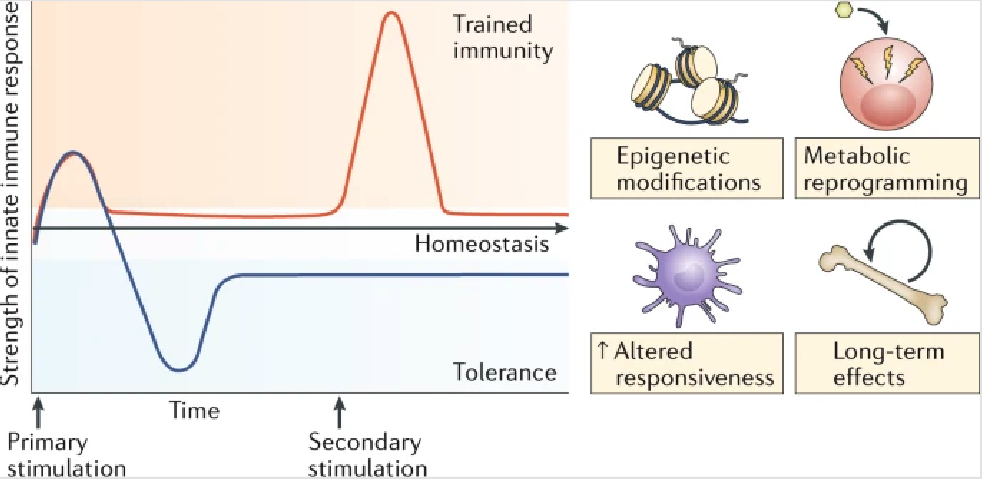
trained immunity
innate immune system can have aspects of memory, but this is antigen-independent
involves epigenetic and metabolic reprogramming of the innate immune cells, allowing qualitatively and quantitatively adjusted responses of innate immune cells to subsequent time-delayed heterologous stimulation.
Trained innate immune cells respond more robustly and faster upon secondary stimulation. Training results in increased cytokine production, increased receptor expression, and enhanced phagocytosis and killing.
what are some of the major unanswered questions relating to adaptive immune memory?
what are the protective correlates of lasting immune memory beyond neutralizing Ab, how to these differ for various pathogens and vaccines?
how do T/B cell responses affect the antigen specificity of B cell responses
how can we monitor immune memory to vaxx and infection, do blood responses show what’s in target tissue?
what are some mechanisms of immune evasion?
subvert immune recognition
evade phagocytosis
sabotage cytokine signalling
inhibit Ag presentation
interference with PRR signaling
modulate intracellular pathways (eg. Ub-proteasome)
short-circuiting cell death pathways
what are some unmet vaccine needs?
no approved vaccine for: RSV, Shigella, ETEC, H. pylori, HIV, HepC
suboptimal vaccine coverage for: M. TB, SARS-CoV2, S. pneumoniae, B. pertussis, H. influenzae
what impact does the route of administration have on vaccination
the route → oral, nasal, intramuscular, subcutaneous, etc. determines the location of immune priming, and different routes can elicit better immune responses than others
IM often preferred for ease of administration and low risk for adverse reactions at site of injection
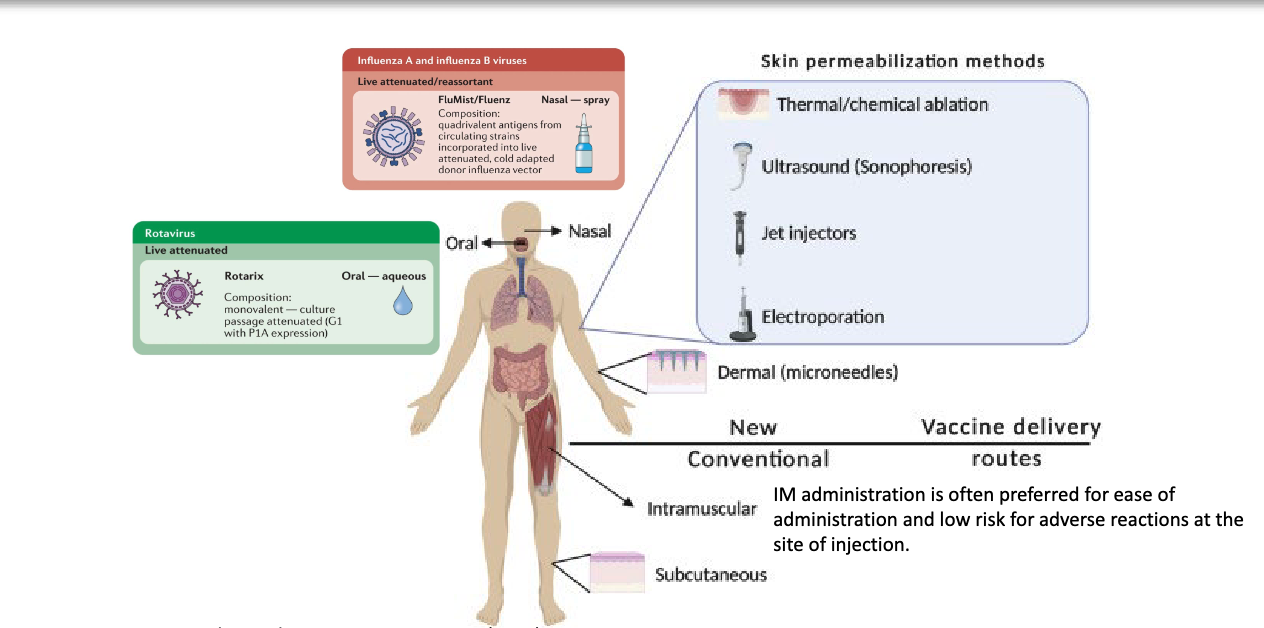
describe examples ff route of administration impacting immune response to vaccine
when using rhesus macaque model, authors identified that when immunizing with nanoparticle, the tissue dissemination was route-dependent, as it was detectable in more LNs when admisistered subcutaneously than IM, but that innate and adaptive responses were the same
location determines priming
animal studies showed that intranasal immunization enhances long lasting anti-viral responses across different SARS-CoV2 variants → more dimer mucosal IgA Ab
describe the response to immunization via the oral route
Oral immunization induces germinal center (GC)-dependent and GC- independent IgA
Generates long lived plasma cells (LLPCs) in the lamina propria (LP)
Antigen-specific GC B cells undergo IgA class-switching in mesenteric lymph nodes (mLN) and the subenthelial dome (SED) of Peyer’s patches (PP)
oral immunization is complicated because already many bacteria in the gut
describe the two proteins targetted in the influenza vaccine
Hemagglutinin (HA)
key role in viral entry and membrane fusion
Immunodominant → we mount strong Ab against it
More abundant than NA - outnumbers NA on the virion surface 4–5:1
Neuraminidase (NA)
critical for the release of newly formed viral particles from infected cells
Ab response to NA is poor (~18% seroconversion rate) vs HA (84%)
less abundant
what stages of life cycle do Abs against HA and NA target?
HA-specific Abs block attachment/entry, while NA-specific Abs target release/dissemination
antigenic shift vs antigenic drift
antigenic shift: reassortment of gene segments between human and/or zoological influenza viruses → dimeric viruses
antigenic drift: point mutations, often in HA globular head domain, reduces vaccines effectiveness from year to year
eg. Influenza A, B, SARS-CoV2
what impacts the mutation rates of a virus
virus-intrinsic characteristics, such as enzymatic error rate and proofreading mechanisms, and emergent and host-dependent properties, such as infection rate. Given equivalent viral-intrinsic characteristics, a more infectious virus will accumulate more mutations in the population as a result of more opportunities to stochastically land on desirable variants.
broad spectrum vaccine
must induce reactivity not only to a limited number of included strains but also to a large and increasing number of (possibly unknown) variants that acquire genetic diversity through myriad molecular mechanisms
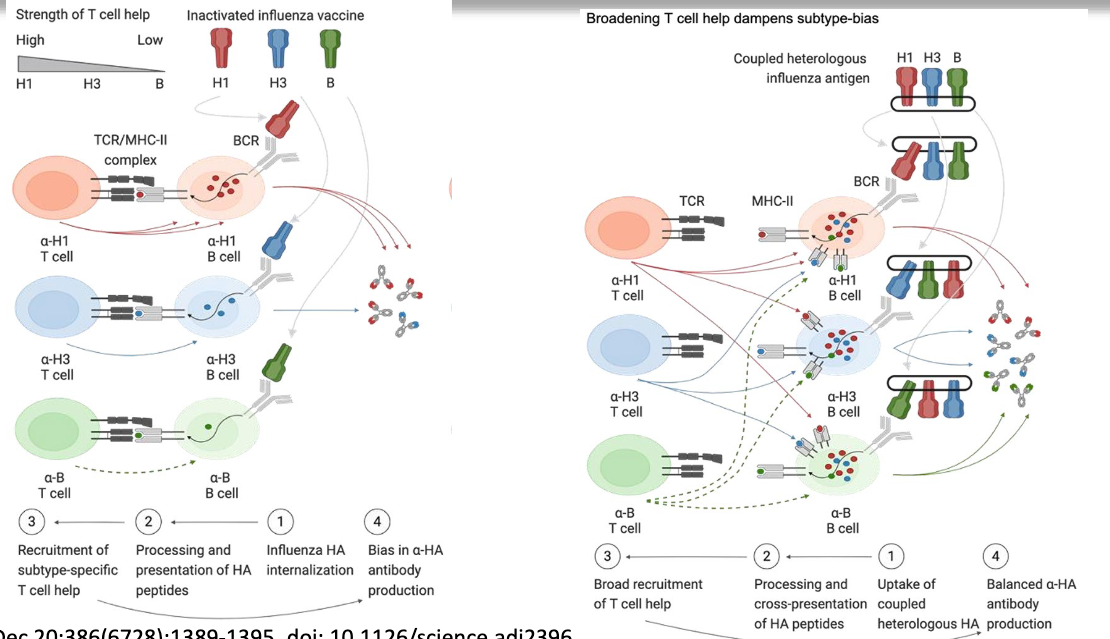
what did the authors observe when the inactivated HA antigens from different subtypes were given separately? how did they fix this?
when given separately, found people developed subtype bias. Influenza HA was internalized and peptides presented on MHC, and got recruitment of subtype-specific T cell help, leading to bias in anti-HA production
coupled the antigens on a scaffold, all get processed and get cross-presentation of HA peptides, leading to broad recruitment of T cell help (B cells get more T cell help), make broader repertoire of antibodies produces
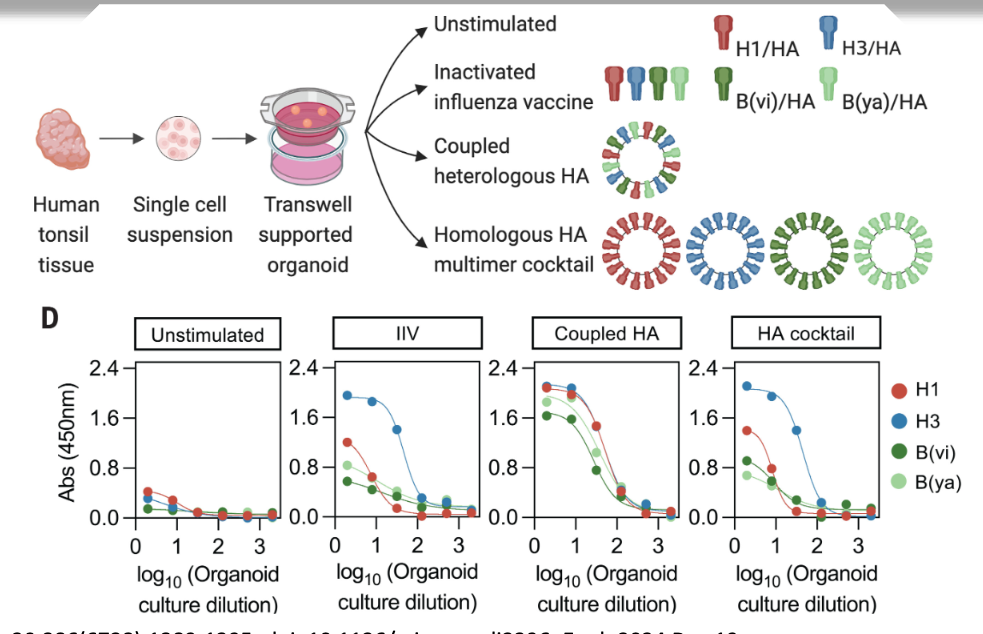
what does this data show?
find that when stimulated with the mix of inactivated influenza vaccine or the homologous HA multimer cocktail, get bias in antibody production → organoids prefer to make either blue, green, etc.
when given the coupled heterologous HA multimer cocktail, subtype bias is not seen
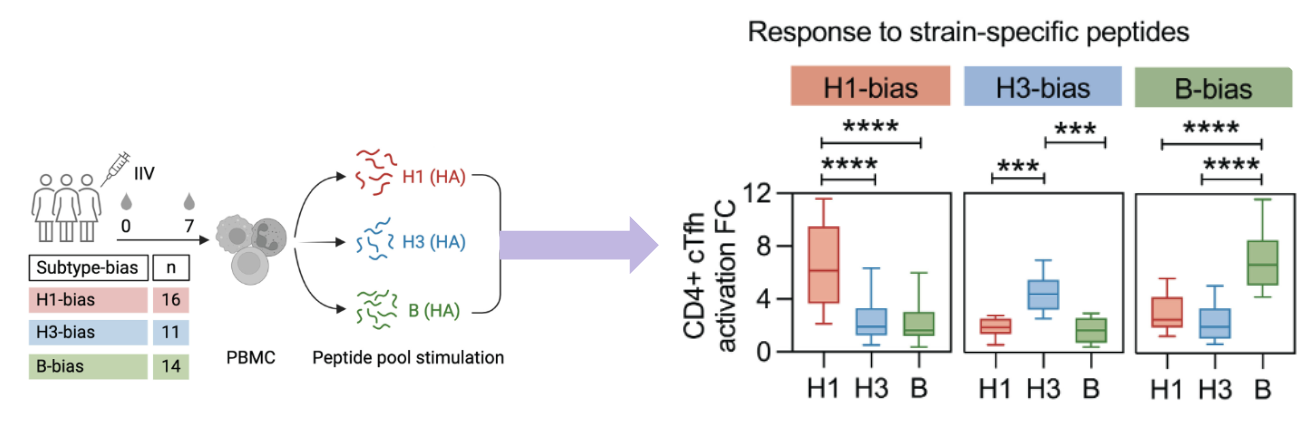
how did they examine what causes bias in host response?
grouped people by bias (peopled with stronger Ab to certain subtype). they then isolated peripheral T cells and stimulated them with peptide pools for each subtype, and found that there was a corresponding T cell bias to the same peptide as the antibody peptide. This could indicate that the T cell bias drives the Ab bias
what factors determine T cell immunodominance?
dependent on precursor frequency - the abundance of epitope-specific T cells in naive repertoire
dependent on antigen presentation
peptide/MHC binding stability: stronger binding = more stable = more immunodominant
antigen dose: how/where Ag expressed/ presented
what factors determine B cell immunodominance
precursor frequency
antigen affinity → how strong is binding? is Ag affinity sufficient for competitive success?
antigen avidity: can antigen be multimerized easily? what is the orientation of the desired epitope on the multimer
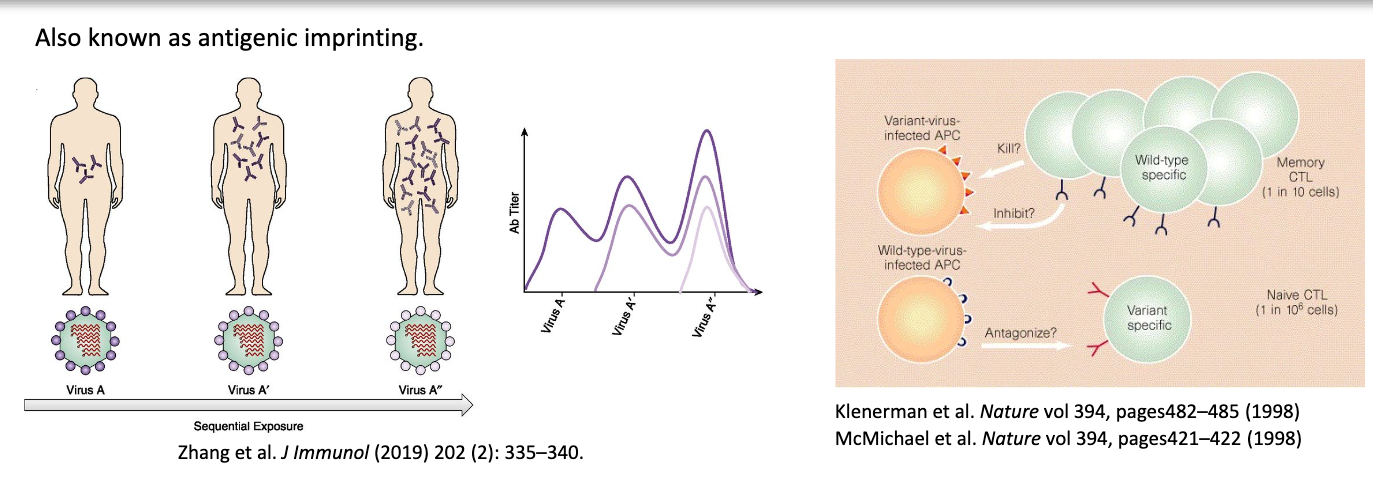
original antigenic sin
also known as antigenic imprinting → refers to tendency of IS to preferentially recall and expand memory B cells generated by first exposure, even when later exposure involves related but antigenically distinct virus
old response dominates response against new antigen
in influenza virus, after initial infection, reinfection (or vaccination) with a new strain of the virus boosted the concentration of antibodies specific for the earlier infecting strain
is original antigenic sin an advantage or disadvantage?
both:
disadvantage as might not be protective against new strain
advantage as if protective, gives very strong/quick response
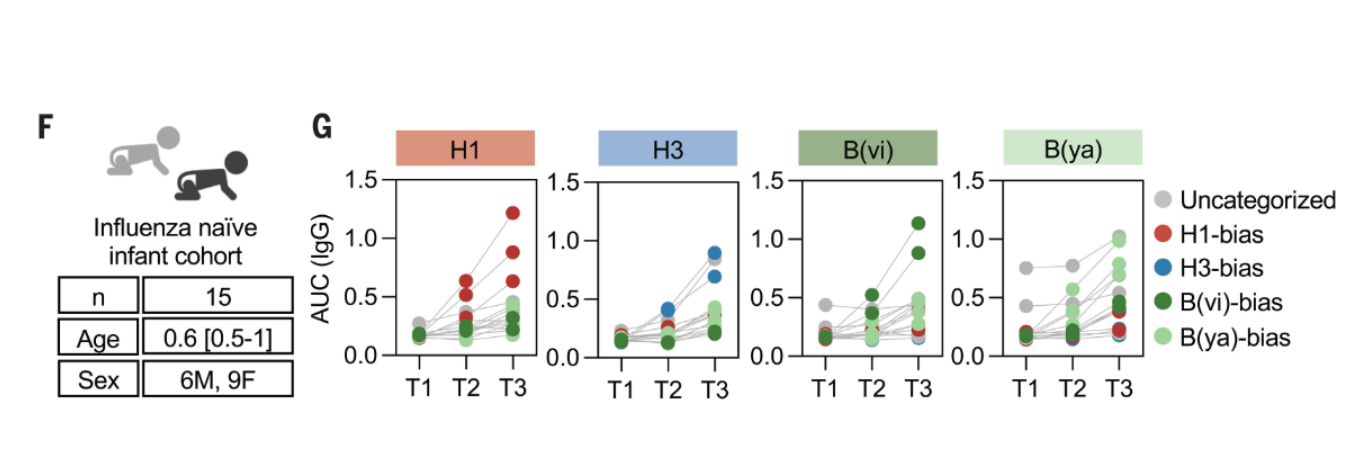
how did they study immune bias in a naive host?
examined Ab levels in babies following immunization who had not previously been immunized or infected → found that without previous exposure, still get bias in a naive population
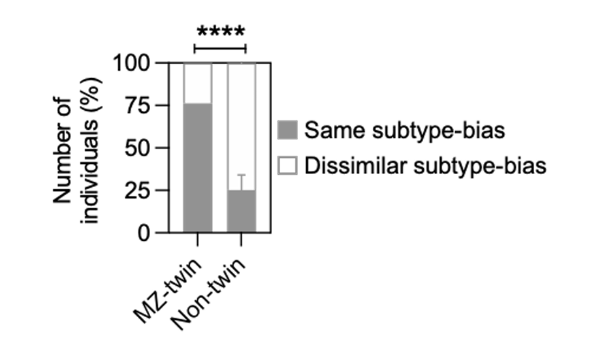
how did they study the host genetic factors involved?
examined monozygotic (identical) and non-twins (have diff MHC) and looked at which proportions had higher same subtype bias vs dissimilar subtype bias
found that much higher proportion of MZ twins had the same subtype bias, indicating a strong genetic factor
how does age impact immune heterogeneity?
finds that immunity and heterogeneity wanes with age → aging alters immune cell development and function, leading to impaired vax response and a senescent phenotype where they produce pro-inflammatory cytokines without being helpful
does biological sex have an impact on immunity
yes → chromosomal factors, differences in gut microbiome, etc
what effect does obesity have on severe respiratory infections and what are the key features of vaccine responses in obesity?
more likely to be hospitalized
key features:
BCR repertoire changes at baseline
defective Ag presentation
skew towards auto-reactive Abs rather than pathogen-specific Abs
accelerated waning of Abs
what are the key take-home messages?
a vaccine works by potentiation Ag-specific immunological memory
type of vax, Ag, and mode of delivery all play a role in determining the type of vaccine response elicited
host factors significantly impact vaccine outcomes
overcoming challenges in vaccine design remains top priority in global pandemic research