C1.2 Cell Respiration
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
What is ATP, & what is it composed of?
ATP (adenosine triphosphate) is a nucleotide composed of:
Nitrogenous base - Adenine
Pentose sugar - Ribose
3 phosphate groups

Where is ATP stored?
ATP is stored in the bonds between phosphate groups
Especially high-energy bond between 2nd & 3rd phosphate (terminal bond)
What properties make ATP ideal? (5)
Property | Why It’s Useful |
Small & soluble | Easily moves around inside cells |
Releases energy in small amounts | Prevents waste of energy as heat |
Rapid breakdown & reformation | Can be recycled quickly & reused |
Universal molecule | Used in all cell types, across all life forms |
Couples w/ many reactions | Drives both anabolic & catabolic processes |
State the processes requiring ATP
1) Active transport across membranes
2) Anabolic reactions (building macromolecules)
3) Movement within or by cells
ATP-powered process: Describe ATP’s role in active transport, & give an example (3)
In active transport, ATP provides energy for carrier proteins (pumps) that move substances against their concentration gradient
Without ATP, substances would only move passively (with the gradient)
Example: Sodium-potassium pump in nerve cells
ATP-powered process: Describe ATP’s role in anabolic reactions, & give examples (4)
ATP powers condensation reactions that build complex molecules from smaller ones
Examples:
Protein synthesis (from amino acids)
DNA/RNA synthesis (from nucleotides)
Glycogen synthesis (from glucose)
ATP-powered process: Give examples of ATP’s role in movement within/by cells (4)
Type of Movement | ATP Role |
Chromosome movement | Powers spindle fibers during mitosis & meiosis |
Cytoplasmic streaming | Helps move organelles through the cytoplasm |
Muscle contraction | ATP is needed to detach myosin heads from actin |
Flagella/cilia movement | Example: Sperm movement
|
What does ADP stand for?
Adenosine diphosphate
What does Pi stand for?
Inorganic phosphate
Describe how ATP releases & stores energy (8)
1) Release - Hydrolysis reaction:
ATP → ADP (adenosine diphosphate) + Pi (inorganic phosphate) + Energy
A water molecule is used to break the bond between last two phosphate groups
Energy is released & used immediately for cellular processes
2) Store - Synthesis (Phosphorylation) reaction:
ADP + Pi + Energy → ATP
Energy is used to rejoin the phosphate group to ADP
This energy is stored in the new ATP molecule
Define cell respiration
A controlled release of energy from organic compounds in cells, used to produce ATP
Describe the process of cell respiration (3)
Carbon compounds act as substrates that are broken down in a series of enzyme-catalysed steps:
Principal substrates: Glucose & Fatty Acids
Others (e.g. proteins)
Energy released from these reactions is used to convert ADP + Pi into ATP
In aerobic cell respiration, ATP is produced w/ CO2 & H2O as waste products
Describe the differences between cell respiration & gas exchange (4×2)
Feature | Cell Respiration | Gas Exchange |
What it is | Chemical reactions that release energy | Physical process of moving gases in/out of cells |
Purpose | To make ATP | To supply O₂ & remove CO₂ |
Location | Cytoplasm & mitochondria | Across cell membranes (e.g., lungs, leaves) |
Gases used/produced | Uses O₂ (aerobic) & produces CO₂ | Brings in O₂ & expels CO₂ |
What is the word equation for aerobic respiration?
Glucose + oxygen → carbon dioxide + water + energy (ATP)
What is the word equation for anaerobic respiration?
Glucose → lactic acid + energy (ATP)
Describe the differences between aerobic & anaerobic cell respiration in humans (5×2)
Feature | Aerobic Respiration | Anaerobic Respiration |
Oxygen required? | ✅ | ❌ |
Main substrate | Glucose (can also use fatty acids, amino acids) | Glucose only |
ATP yield | High (~36–38 ATP per glucose) | Low (only 2 ATP per glucose) |
Waste products | Carbon dioxide (CO₂) + water (H₂O) | Lactic acid (lactate) |
Where in the cell? | 1. Starts in cytoplasm (glycolysis) 2. Continues & finishes in mitochondria (Krebs cycle + electron transport chain) | Entirely in cytoplasm (no mitochondria needed) |
What does the rate of cell respiration refer to, & how is it measured? (6)
How quickly a cell produces ATP by breaking down organic compounds (glucose or others)
It is often measured by:
Oxygen consumption
Carbon dioxide production
Change in pH
Heat released
Describe the methods of measuring rate of cell respiration
Method | Factor measured |
Respirometer (with seeds or insects) | Volume of oxygen consumed |
CO₂ probe | Rate of carbon dioxide production |
pH meter in yeast/glucose solution | Drop in pH as CO₂ forms carbonic acid |
Calorimeter | Heat produced by respiring organisms |
Describe how rate of cell respiration varies with different variables
Variable | Effect |
Temperature |
|
pH | Each enzyme has an optimal pH. Deviations slow down or stop respiration. |
Oxygen availability | Limited oxygen shifts cells from faster aerobic to slower anaerobic |
Glucose concentration | More glucose = more fuel for higher rate (up to a saturation point) |
Enzyme concentration | More enzymes = faster reaction, as long as substrate is available |
Cell type or tissue type | Some cells (like muscles) have more mitochondria = higher respiration rates |
What is NAD & its forms? (4)
Nicotinamide Adenine Dinucleotide (NAD) is a coenzyme used in cell respiration to carry hydrogen atoms from one reaction to another
It exists in two forms:
NAD⁺ → oxidized form (ready to accept hydrogen)
NADH → reduced form (carrying hydrogen)
Define redox reaction
A reaction where one substance is oxidized and another is reduced
What redox reactions are involved in respiration?
1) Oxidation | Substrate loses electrons or hydrogen (often via dehydrogenation) → this is carried out by enzymes called dehydrogenases |
2) Reduction | Coenzyme gains electrons or hydrogen |
Describe NAD’s role in respiration (4)
NAD picks up hydrogen atoms removed from substrates (e.g. glucose intermediates)
Accepts 1 H⁺ ion & 2 electrons → reduced into NADH
NADH carries these electrons to the electron transport chain in mitochondria
In the final step, NADH is oxidized back to NAD⁺, releasing energy to make ATP
What is glycolysis? State its metabolites
⭐ The first stage of cellular respiration
Role: A linear pathway of enzyme-catalysed steps breaking down glucose (6-carbon) into 2 pyruvate molecules (3-carbon)
There is a net gain of:
2 ATP molecules
2 NADH molecules (reduced NAD)
Location: Cytoplasm
——————————————————————————————————
What’s involved (Metabolites) -
Starting substrate: Glucose
Intermediate: Pyruvate
Products (net gain): 2 Pyruvate, 2 ATP molecules, 2 NADH (reduced NAD⁺)
Describe the stages of glycolysis (4)
Stage | What Happens |
1. Phosphorylation | Glucose is phosphorylated by using 2 ATP molecules |
2. Lysis | The phosphorylated glucose splits into two 3-carbon sugars |
3. Oxidation | Each 3-carbon molecule is oxidised, causing H atoms to be removed
|
4. ATP Formation |
|
Explain what happens to respiration in low oxygen conditions
In low oxygen conditions, cells switch from aerobic to anaerobic respiration
Glycolysis still occurs but w/o oxygen, NADH can’t offload hydrogen via the electron transport chain
As a solution, pyruvate is converted into lactate in the cytoplasm (lactic acid fermentation)
Describe the process of lactic acid fermentation
Step | Purpose |
1. Pyruvate is reduced to lactate | Accepts hydrogen from NADH (via lactate dehydrogenase) |
2. NADH is oxidised to NAD⁺ | Regenerates NAD⁺ so glycolysis can continue |
Compare anaerobic respiration in humans vs yeast
Anaerobic respiration is largely similar in humans & yeast
The difference lies in how NAD⁺ is regenerated & its final products:
Organism | Pyruvate is converted to… | Final products |
Humans | Lactate | Lactate (lactic acid) |
Yeast | Ethanol + CO₂ | Ethanol (alcohol) + carbon dioxide |
What is the link reaction? State its metabolites (4)
⭐ The second stage of cellular respiration
Role: A series of enzyme-catalysed steps connecting Glycolysis to the Krebs Cycle
Location: Mitochondrial matrix
Only occurs under the presence of oxygen (aerobic conditions)
——————————————————————————————————
What’s involved (Metabolites) -
Starting substrate: Pyruvate
Intermediate: Acetyl group
Products:
Final: Acetyl-CoA
Byproducts: CO₂ , 2 NADH
Describe the process of the link reaction
Stage | What Happens |
1. Decarboxylation |
|
2. Oxidation |
|
3. Coenzyme A (CoA) |
|
How do carbohydrates vs lipids proceed to Krebs cycle?
Both carbohydrates & lipids form acetyl-CoA & proceed to Krebs cycle
However, only carbohydrates undergo link reaction
Describe how carbohydrates undergo cell respiration until Krebs Cycle (4)
Glucose is broken down by glycolysis in the cytoplasm to form pyruvate (3C)
In the link reaction, pyruvate is decarboxylated & oxidised to form an acetyl (2C) group
This acetyl group combines w/ coenzyme A to form acetyl‑CoA
This acetyl‑CoA feeds into Krebs cycle
Describe how lipids undergo cell respiration (4)
Lipids are broken into fatty acids, which are transported to mitochondria
Fatty acids are oxidised at matrix, breaking it into multiple acetyl (2C) units
Each 2C acetyl unit combines w/ coenzyme A to form acetyl‑CoA
This acetyl‑CoA feeds into Krebs cycle
Describe the process of the Krebs cycle
Acetyl-CoA (2C) combines w/ oxaloacetate (4C) → forming citrate (6C)
Citrate undergoes a series of reactions that:
Release 2 CO₂ molecules (decarboxylations)
Perform 4 oxidations (dehydrogenation reactions)
Regenerate oxaloacetate (4C) to restart the cycle
What type of reaction is oxidation
Dehydrogenation reactions = hydrogen atoms are removed from intermediates
What is the Krebs Cycle? State its metabolites
⭐ The third stage of cellular respiration
Role: A cyclical pathway of enzyme-catalysed reactions that oxidises acetyl-CoA to release ATP
Location: Mitochondrial matrix
——————————————————————————————————
What’s involved (Metabolites) -
Starting substrate: Acetyl-CoA (2C)
Intermediates: Citrate (6C), Oxaloacetate (4C)
Products:
Final: 6 NADH (3 per cycle), 1 FADH2, 1 ATP
Byproduct: 2 CO2
Describe the process of the Krebs Cycle (8)
Acetyl-CoA (2C) combines w/ oxaloacetate (4C) → forming citrate (6C)
Citrate undergoes a series of reactions that:
Release 2 CO₂ molecules (decarboxylations)
Perform 4 oxidations (dehydrogenation reactions), which produce:
3 NADH
1 FADH2
1 ATP
Oxaloacetate (4C) is regenerated for 2nd cycle
What are the stages of cell respiration?
1) Glycolysis
2) Link reaction
3) Krebs Cycle
4) Oxidative Phosphorylation: Electron transport & chemiosmosis
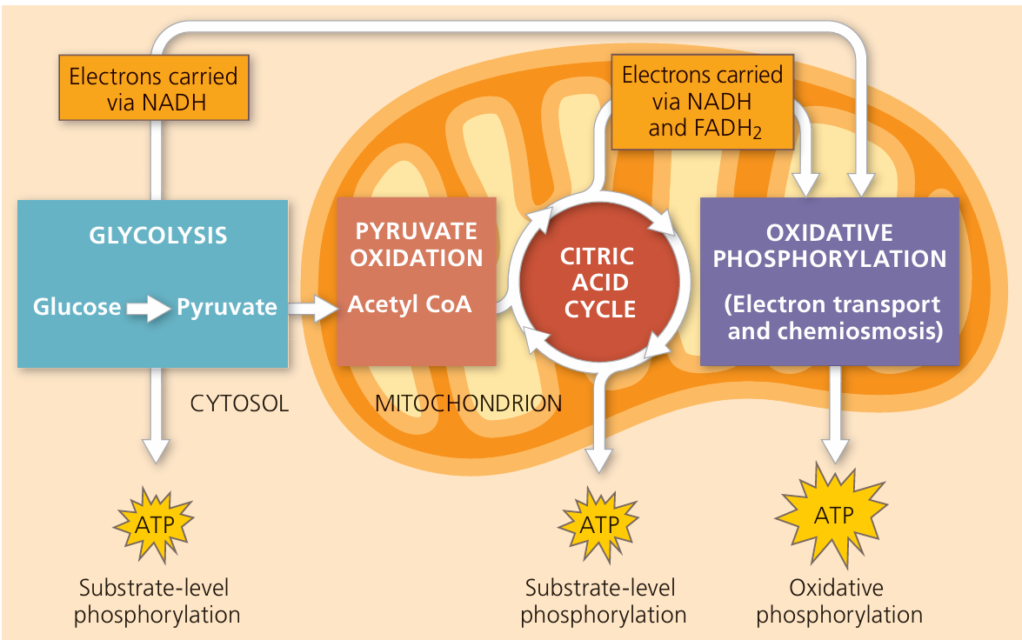
What is the role of NADH?
Reduced NAD (NADH) carries electrons & hydrogen down the electron transport chain
It is provided & accumulated from previous stages of cell respiration
Process | NADH produced |
Glycolysis | 2 NADH per glucose |
Link Reaction | 2 NADH per glucose |
Krebs Cycle | 6 NADH per glucose |
Total from one glucose = 10 NADH | |
Describe the process of NADH transfer down the ETC (5)
NADH donates a pair of electrons to the first carrier in the ETC
This oxidizes NADH → converting back to NAD⁺, which can be reused
Electrons move along the chain, releasing energy at each step
This energy is used to pump H⁺ ions (protons) into the intermembrane space
This sets up a proton gradient needed for ATP synthesis via chemiosmosis
Define proton gradient
A difference in H⁺ (proton) concentration across a membrane
Describe the process of proton gradient formation
NADH and FADH₂ donate electrons to the electron transport chain (ETC)
As electrons flow along the ETC, they release energy
This energy is used to pump protons (H⁺) from the matrix into intermembrane space
This results in:
High concentration of protons in the intermembrane space
Low concentration in the matrix
Define chemiosmosis
The movement of protons (H⁺) down their concentration gradient across a membrane through ATP synthase
Describe the features of chemiosmosis (5)
⭐ Along w/ the ETC, it makes up the final stage of cell respiration
Role: Links energy released by the ETC to the phosphorylation of ADP → producing ATP
Location: Inside mitochondria, specifically:
Across the inner mitochondrial membrane
Between the intermembrane space & matrix
Describe the process of ATP synthesis (oxidative phosphorylation)
The ETC pumps H⁺ ions into the intermembrane space, creating a proton gradient
Protons flow back into the matrix through ATP synthase (a membrane protein)
The energy from this proton movement is used by ATP synthase to:
Add a phosphate group to ADP → forming ATP (adenosine triphosphate)
What is oxygen’s role in cell respiration?
Oxygen is the terminal electron acceptor in cell respiration
At the end of the ETC, it combines with:
Electrons coming from the ETC
Protons from the matrix
This forms metabolic water & allows continuous flow of electrons along ETC
What happens if oxygen is absent in cell respiration? (3)
Electrons have nowhere to go so they back up the ETC
The ETC stops = no more proton pumping
ATP production halt
Define respiratory substrate
Molecules used to release energy in cell respiration
What are the main types of respiratory substrate?
1) Carbohydrates (like glucose)
2) Lipids (like triglycerides → fatty acids)
Compare cell respiration with carbohydrates vs lipids (6)
Feature | Carbohydrates | Lipids |
Energy yield /g | Lower | Higher |
Oxygen content | Higher | Lower |
Hydrogen & carbon | Less oxidisable | More oxidisable |
Pathway entry | Glucose → Pyruvate → Acetyl-CoA | Fatty acids → 2C acetyl groups → Acetyl-CoA |
Used in glycolysis? | ✅ | ❌ |
Used in anaerobic respiration? | ✅ | ❌ |
Why do lipids have higher energy yields
1) Have more C-H bonds to be oxidised during respiration
2) They are also less oxygenated → more energy released when oxygen added