Chemistry - Unit 12 - Collision Theory and Equilibrium
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
Collision Theory
In order for a chemical reaction to occur a collision must occur between the particles, with the correct orientation and the correct speed.
What reacts faster, ionic or covalent compounds and why?
Ionic compounds generally react faster, easier to separate into ions than break covalent bond
Aqueous or solid react fasters?
Aqueous solution react faster than solid because the compound is already mobile
What group reacts the fastest?
Group 1 metals react faster, lower ionization energy
Do noble gases react fast?
Most elements/compounds react faster than noble gases
Collision Theory and Reaction Rates
Ionic compounds generally react faster, easier to separate into ions than break covalent bond
Aqueous solution react faster than solid because the compound is already mobile
Group 1 metals react faster, lower ionization energy
Most elements/compounds react faster than noble gases
THIS IMPACTS REACTION RATES OF THE FORWARD AND REVERSE REACTION
Factors that can Increase Reaction (AND WHY)
Temperature: particles have more KE, move more, which leads to more collisions
Concentration: if it is more concentrated, there are more particles in the same space, leads to more collisions
Pressure: increase pressure (decrease volume) if there is less space for particle to move they will collide more
Surface Area: if there is more surface where the reaction can happen then are more chances for a successful collision
Catalyst: lowers activation energy, makes it easier for reaction to happen
How does adding a catalyst increase reaction rate?
Adding a catalyst lowers activation energy, making it easier for reaction to occur. It does NOT impact equilibrium. It also changes the path of the reaction.
Chemical Equilibrium
when the rate of the forward reaction = the rate of the reverse reaction
amount/concentration of reactants and products is constant
This is also a DYNAMIC EQUILIBRIUM: Reaction is STILL happening in forward AND reverse direction but at the same time
What is dynamic equilibrium? (give two examples)
Reaction is STILL happening in forward and reverse direction but at the same time
Dynamic Solution Equilibrium: In a saturated solution at equilbiruim (constant temp) the rate of dissolving equals the rate of crystallization
Dynamic Phase Equilibrium: In a closed system at equilibrium with a substance in liquid and solid state (constant Temp): Rate of evaporation = rate of condensation
Rate of freezing = rate of melting
Rate sublimation = rate of deposition
Static equilibrium
When the reaction completely stops after equilbrium is reached
What is solution equilbirum? (Conditions, molecules, dynamic or static)
In a saturated solution at equilibrium (constant Temp): the rate of dissolving = rate of crystallization
DYNAMIC because the chemical reaction continues to occur
CLOSED SYSTEM
Phase Equilibrium
the state where two or more phases of matter (solid, liquid, gas) coexist in a stable manner (constant temp):
Rate of evaporation = rate of condensation
Rate of freezing = rate of melting
Rate sublimation = rate of deposition
**CLOSED SYSTEM!!**
Occurs at boiling point and melting point at standard temperature becuase
Boiling point = vapor pressure is equal to atmospheric pressure —> G and L coexist
Melting point = rate of melting is equal to the rate of freezing —> S and L coexist
What is sublimation and deposition
Sublimation is the phase transition where a substance changes directly from a solid to a gas, skipping the liquid phase, while deposition is the reverse process, where a substance changes directly from a gas to a solid
What type of system is needed for equilibrium to be reached and why?
A CLOSED SYSTEM
—> A closed system is required for chemical equilibrium because it prevents the loss or gain of matter, allowing the forward and reverse reaction rates to balance and establish a stable state where concentrations remain constant.
Equilibrium (Defenition and how to tell it is at equilibrium)
Chemical Equilibrium: when the rate of the forward reaction = the rate of the reverse reaction (exchanging the same amount of beads)
amount/concentration of reactants and products is constant
must be a closed system to achieve equilibrium
Only reversible reactions (<-->) can reach equilibrium
Phase equilibrium: constant temp, same substance changing phases
Solution equilibrium: constant temp, equal rate of dissolve/crystalize
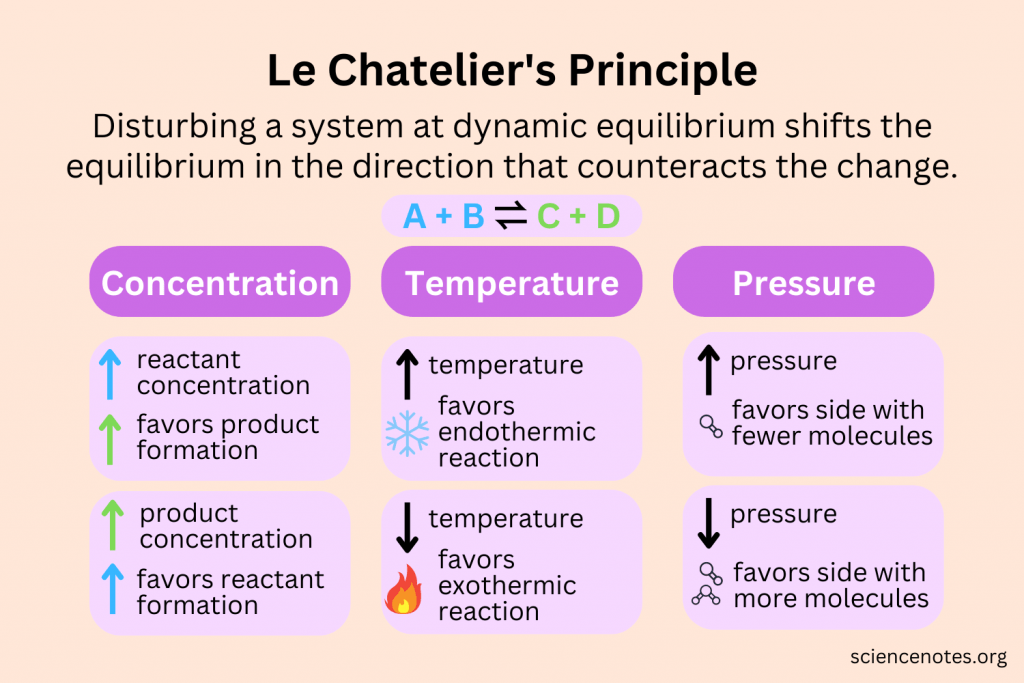
Changes in Equilibrium - Le Châtelier’s Principle
A system at equilibrium will respond to reduce stress by increasing forward (-→) reverse (<--) reaction to re-establish equilibrium
will shift equilibrium (what needs to change) to get system back to equilibrium
if forward (-->) rxn increase the reverse (<--) decreases
Stress can be
Change in concentration
Change in temperature
Change in concentration/volume
How does the reaction/Equilibrium shift when reactant is increased?
More reactant = more things to be new compounds
How does the reaction/Equilibrium shift when reactant is dereased?
Less things to make the new compounds also, less concentration = lower rate of reactions
How can you tell if something is in phase equilibrium?
A substance is in phase equilibrium when multiple phases (solid, liquid, gas) coexist and the amount of material in each phase remains constant, even though molecules are constantly moving between them.
Here's a more detailed explanation:
Definition:
Phase equilibrium refers to a state where two or more phases of a substance exist simultaneously and the system is in a dynamic state, meaning there's constant movement between phases, but no net change in the amounts of each phase.
Conditions:
Equal Temperature and Pressure: All phases in equilibrium must have the same temperature and pressure.
Equal Chemical Potential: The chemical potential (a measure of the tendency of a substance to change phase) of each component must be the same in all coexisting phases.
Minimum Gibbs Free Energy: The overall Gibbs free energy of the system must be at a minimum.
Examples:
Water: Liquid water and water vapor in a closed container at the boiling point are in equilibrium.
Ice and Water: Ice and water at 0°C (or 32°F) and 1 atmosphere pressure are in equilibrium.
Triple Point: The triple point is a specific temperature and pressure where all three phases (solid, liquid, and gas) can coexist in equilibrium.
What is the impact of pressure on reversable reactions?
When volume is decreased more NO2 (brown) in a smaller space so the color is more intense.
This is while the reaction is adjusting, before it gets back to equilibrium.
When the volume decrease the particles are in a smaller space together.
Because they are in a smaller space, more collisions occur
More collision favors 2NO2→N2O4 (colorless product)
The system wants to release pressure, prefers the reaction that produces less gas
How does a gas equilibrium system change when P increases/V decreases
ONLY IMPACTS GAS
When pressure increases the system will shift to the side with less gas particles
The system needs to reduce pressure somehow, easiest way to do that is decrease the number of gas particles, so it shifts to the side with less gas particles.
What does pressure change impact?
ONLY IMPACTS GASES WHICH ARE COMPRESSIBLE DUE TO PARTICLE ARRANGMENT
When pressure increases the system will shift to the side with less gas particles
Gas Systems at Equilibrium
↓ volume / ↑ pressure P: system equilibrium shifts to less mole of gas (reduce P with less gas particles)
↑ volume / ↓ decrease P: system equilibrium shifts to more moles of gas (increase P with more gas particles)
Le Châtelier’s Principle
A system at equilibrium will respond to reduce stress by increasing forward (-->) or reverse (<--) reaction to re-establish equilibrium
what needs to change to get system back to equilibrium
if forward (-->) rxn increase the reverse (<--) decreases
Stress can be
Change in concentration
Change in temperature
If something is added it must be “used up”
If something is removed it must be replaced
Remember:
AATT → Add Away Take Toward
Label the graph (A,B,C)
Section A = equilibrium because the lines are flat (constant)
Up line (right after Section A) = The stress or change which in this case is additional H2
Section B: Stress and change to equilibrium
Right after = decrease because H2 gets used up
HI increase: more product is created
I2 decreases because it gets used with the additional H2 to create HI
Section C: Return to equilibrium (lines are flat) but now there is more of the product
What are the axis titles for reaction graphs?
Reactants and products = Y axis
Time = X axis
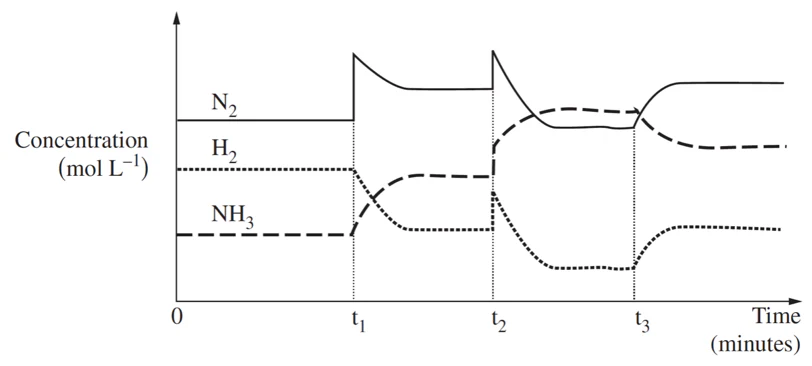
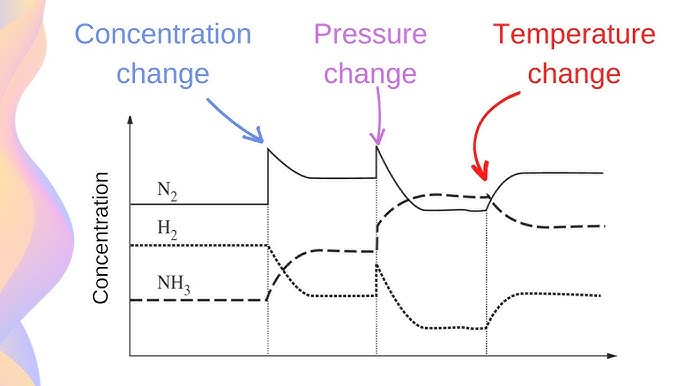
What are the three changes (in order)
Curve change = TEMPERATURE change
Sharp change = changing concentration (by adding or taking away reactants or products)
Could also be pressure change
On an equilibrium graph, concentration changes shift the equilibrium position to counteract the change, while pressure changes, especially in gaseous reactions, shift the equilibrium to favor the side with fewer gas molecules to reduce pressure.
LeChateliers Principle
What does adding a catalyst do (added to system at equilibrium) AND HOW?? (Reaction pathway)
Increases the rate of both forward and reverse reaction
A catalyst increases the rate of both forward and reverse reactions in a reversible reaction by providing an alternative reaction pathway with a lower activation energy, thus accelerating the reaction without altering the equilibrium position
Activation energy: how much energy is needed to break apart bonds before reforming them