Gibbs Free Energy
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 11:54 AM on 7/31/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
What is GFE used for
The feasibility of a reaction is determined by the balance between ΔH and ΔS° which is what GFE is.
2
New cards
Formula for GFE
∆G = ∆H – T∆S
T= ∆H/(∆S/1000)
ALWAYS DIVIDE T∆S BY 1000
If ∆G is -ve the reaction is feasible and vice versa.
3
New cards
When might a reaction not be feasible even when ∆G <0
A very high Ea
A kinetically inert system
4
New cards
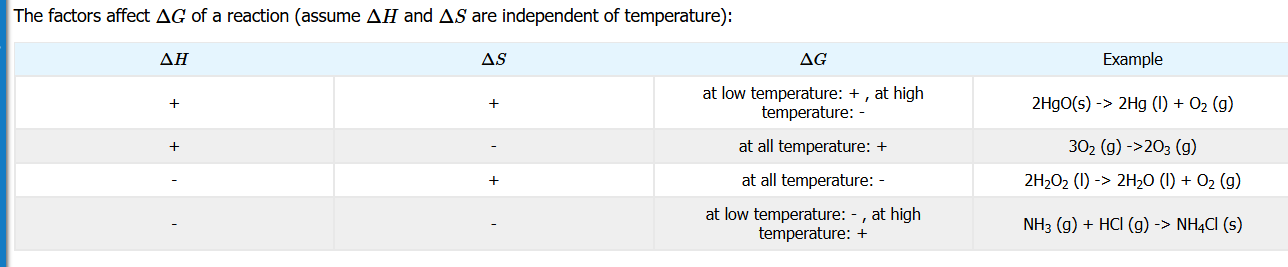
Factors affecting ∆G