Ch. 25 - Body Fluids
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
A living cell requires…
a steady supply of reactants and a reliable system for removing waste
Carried out by diffusion in simple organisms
Complex organisms rely on a circulating system to prevent the depletion of the reactant and stop accumulation of wastes in the cell
Body Fluids
the average adult contains 42 L of fluids, which accounts for 2/3 of the total body weight
located in the following regions of the body:
Interior of cells
Tissue spaces between cells
Blood vessels
Intracellular Fluid
Located inside cells
Majority of body fluid (28 L)
substance where vital life-maintaining reactions occur
Extracellular Fluid
Located outside of cells
Provides a constant environment for the cells and transports substances to and from the cells
Includes:
interstitial fluid
plasma
Interstitial Fluid
fluid that fills the space between tissue cells and moves in lymph vessels
Constitutes about 25% (10.5 L) of the total body fluid
Plasma
Fluid of the bloodstream
makes up about 8% (3.5 L) of the total body fluid
Plasma and interstitial fluid are nearly identical, except plasma has more protein
Other Body Fluids
urine
digestive juices
cerebrospinal fluid
Chemical Differences between ECF and ICF
Intracellular fluid
Principle cation: K+
Principle anion: Phosphate (HPO42−)
Contains four times more protein than plasma
Extracellular fluid
Principle cation: Na+
Principle anion: Cl−
Interstitial fluid contains very little protein and plasma contains high protein
How Oxygen gets Transported:
Oxygen required by the body is mostly carried by red blood cells in the form of oxyhemoglobin
Limited solubility of oxygen in plasma only allows about 2% to be dissolved and transported in solution by hemoglobin
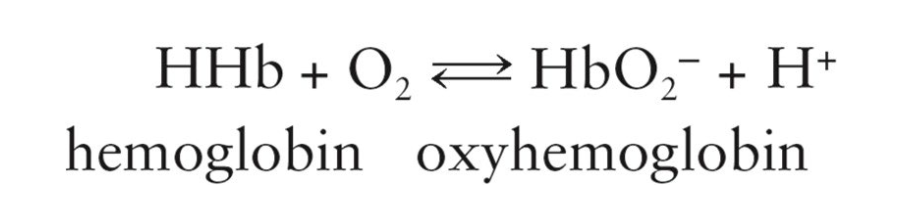
Oxyhemoglobin
Oxygen-hemoglobin combination
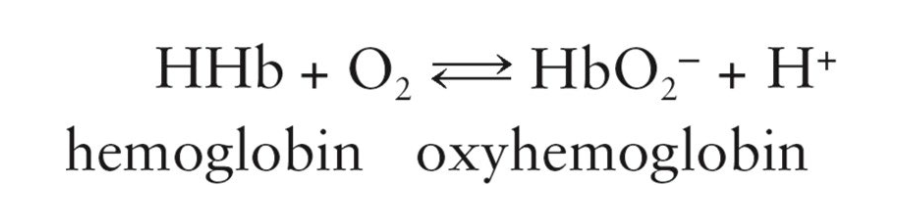
Deoxyhemoglobin (hemoglobin)
Nonoxygenated hemoglobin
How CO2 gets Transported:
When CO2 is present, hemoglobin can reversibly bind to it to form carbaminohemoglobin
CO2 is transported from body tissues to the lungs
25% is carried in the form of carbaminohemoglobin
5% is dissolved in the plasma
70% is transported as bicarbonate ions
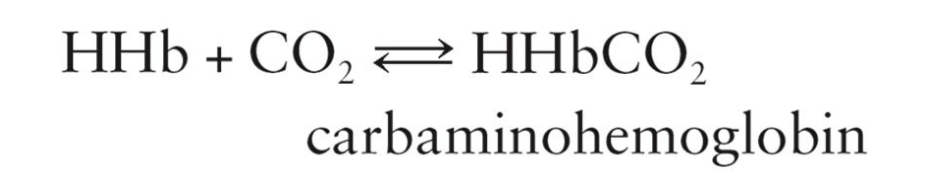
Carbaminohemoglobin
Hemoglobin combined with carbon dioxide
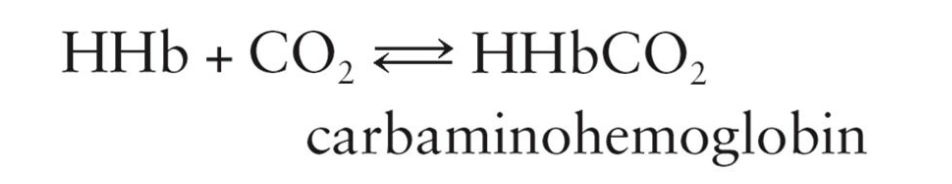
How Oxygen Transports to Tissues (7 Steps):
Oxygen diffuses from the alveoli through capillary wall and into the red blood cells
Oxygen reacts with hemoglobin to form oxyhemoglobin and H+ ions inside the red blood cells
Oxyhemoglobin formation is favored by a high partial pressure of oxygen
Bicarbonate ions diffuse from the plasma into the red blood cells
Replaced in the plasma by chloride ions that diffuse from blood cells
chloride shift
Protons from the oxygenation of hemoglobin react with bicarbonate ions to form carbonic acid
Carbonic anhydrase (enzyme in the red blood cells) promotes the breakdown of carbonic acid to water and carbon dioxide
Formation of CO2 and water is favored by the low pressure of carbon dioxide in the lungs
Carbaminohemoglobin breaks apart to yield hemoglobin and carbon dioxide
Carbon dioxide molecules diffuse out of the red blood cells and into the lung because the CO2 pressure is high in red blood cells and low in the alveoli
Carbon dioxide and some water are expelled in the exhaled air
Chloride Shift
Maintains the charge balance and osmotic pressure relationships between the plasma and red blood cells
How CO2 gets Transported to the Lungs (8 Steps):
CO2 diffuses from the tissue cells into the interstitial fluid and into the red blood cells because of higher concentration of CO2 in tissue cells
CO2 reacts with water in the presence of carbonic anhydrase to produce carbonic acid inside the red blood cells
Carbonic acid dissociates to give hydrogen ions and bicarbonate ions
Bicarbonate ions diffuse into the plasma and transport most CO2 in bicarbonate form from the tissue cells to the lungs
Chloride shift occurs to maintain electrolyte balance
25% of the CO2 from Step 1 reacts with hemoglobin to form carbaminohemoglobin for transport to the lungs
most CO2 is transported in the bicarbonate molecule
Increase of the H ion concentration inside the red blood cells promotes a reaction with oxyhemoglobin, which releases oxygen
Free oxygen diffuses out of the red blood cells through the plasma, capillary membrane, interstitial fluid, and into the tissue cells
Chemical Transport to the Cells:
Substances must become part of the moving bloodstream to be chemically transported
May dissolve in water-based plasma
ex) Sugars and ions
May bind to cellular components
ex) O2 and CO2 with hemoglobin
May form a suspension in the plasma
ex) Lipids
Capillary walls behave as selectively permeable membranes to allow:
Water containing dissolved nutrients (including O2) to pass in one direction
Water containing dissolved wastes to pass in the other direction
Movement of water and dissolved materials is governed by:
Pressure of blood against the capillary walls
Differences in protein concentration on each side of the capillary walls
Protein concentration in plasma is…
higher than the protein concentration in the interstitial fluid outside blood vessels
Results in osmotic pressure
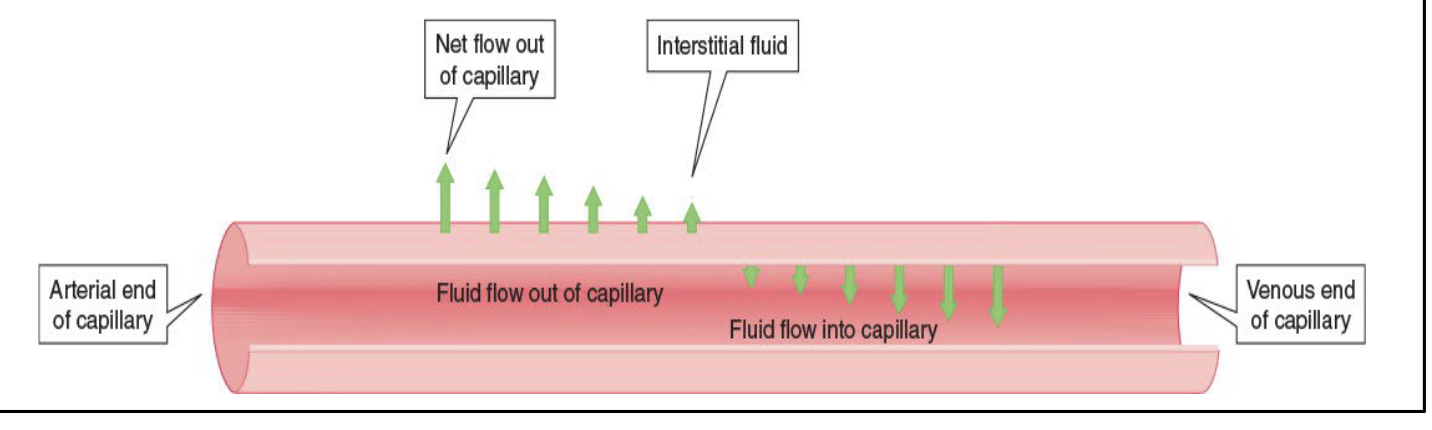
Chemical Transport across Capillaries
The pumping action of the heart creates pressure in the blood that is greater in the arterial end of a capillary than at the venous end
At the arterial end, blood pressure is greater than the osmotic pressure, which results in a net outflow into the interstitial fluid
At the venous end, blood pressure is less than the osmotic pressure, which results in a net inflow from the interstitial fluid
Components of Urine
96% water and 4% dissolved waste products
Approximately 40–50 g of dissolved solids are found in daily urine output of an adult
there is 25g of urea in daily urine output
What is the urine pH of a healthy person?
4.5–8.0
6.6 is a reasonable average for an ordinary diet
Fruits and vegetables make urine basic
High-protein foods make urine acidic
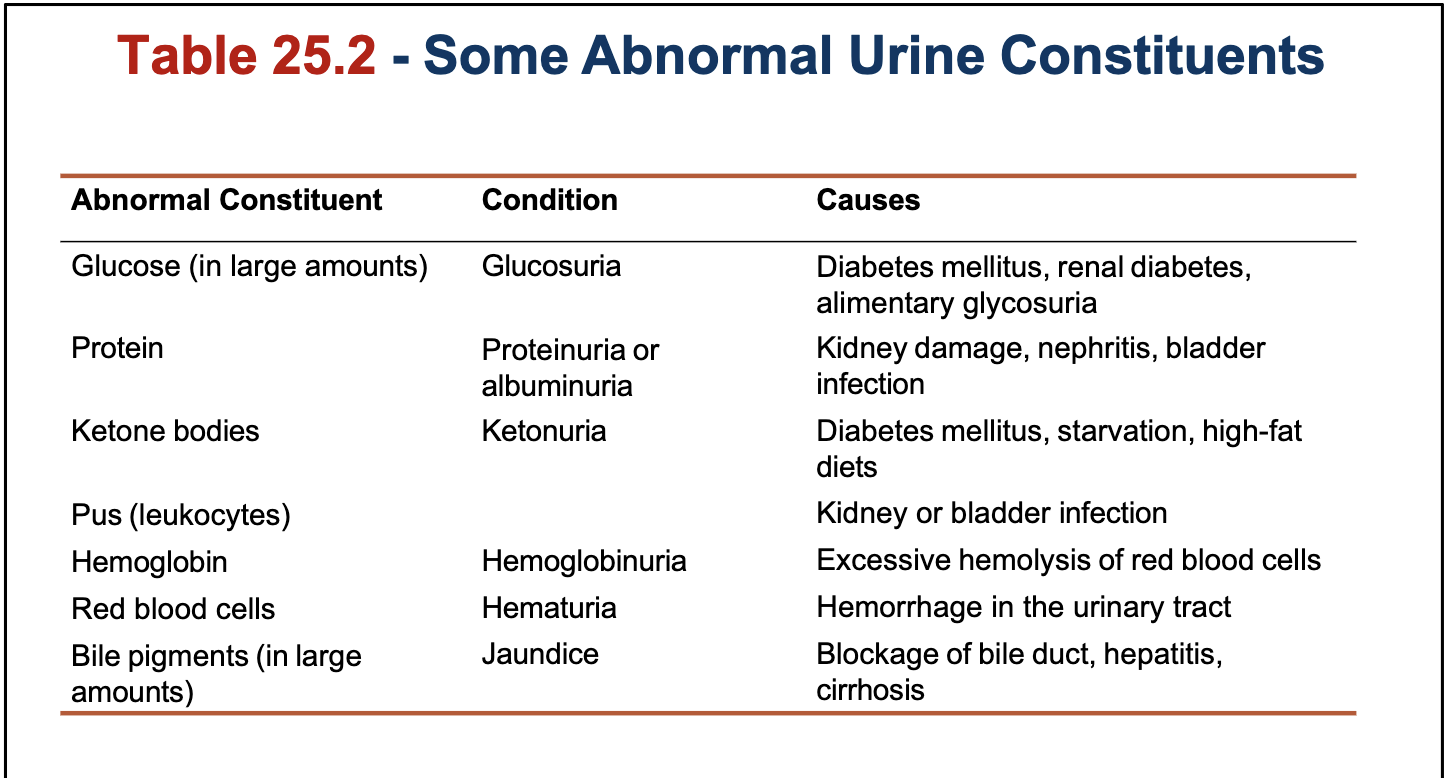
How urine composition can be used for diagnosis:
Urine specimen can be checked with paper test strip
Contains bands of reagents that react with the abnormal components of urine
Quickly checks for indications of pathological conditions
Fluid and Electrolyte Balance
Fluid balance within the body is maintained by:
Balance in the total amount
Normal and stable distribution of fluid inside the cells, in the interstitial spaces, and in the blood vessels
When the fluid balance deviates, the electrolyte balance deviates as well
Fluid output and intake must be equal
Fluid balance is maintained or restored by variations in urine output
Urine output - 1400 mL/day
Thirst Mechanism
Regulates water intake
Stimulated when the body loses large amounts of water, salivary secretions decrease and a dry feeling develops in the mouth
Water leaves the body through the:
Kidneys (urine)
Lungs (water vapor in expired air)
Skin (diffusion and perspiration)
Intestines (feces)
Abnormally high fluid losses, and possibly dehydration, can be caused by hyperventilation, excessive sweating, vomiting, or diarrhea
What controls urine production?
The rate that kidneys reabsorb water
How is urine production regulated?
Regulated by vasopressin (aka the antidiuretic hormone, ADH) and the adrenal cortex hormone aldosterone
Vasopressin regulates urine production by affecting the permeability of the renal tubules to water
When bodily fluid levels run low, what happens?
aldosterone is secreted, which then stimulates the reabsorption of Na +
Chloride ions follow the sodium ions to maintain electrical neutrality, and water follows sodium chloride
Conserves salt and water in the body
Amount of aldosterone secretion decreases when the fluid level is back to normal
Acid-Base Balance
Normal blood pH is 7.35 to 7.45
Death can result when pH value falls below 6.8 or rises above 7.8
Large amounts of acids and smaller amount of bases enter the blood
Constant pH is maintained by the interactive operation of
buffer systems
respiratory systems
urinary systems
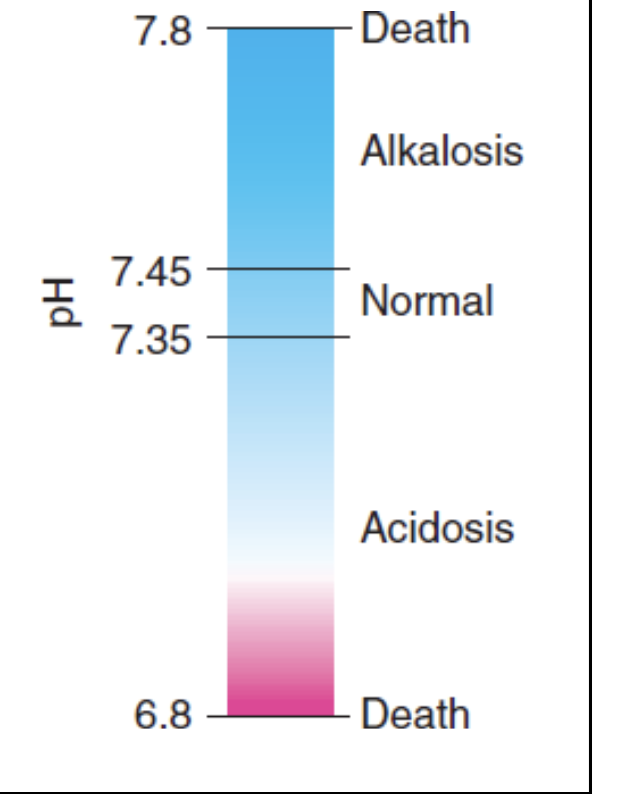
Alkalosis Vs. Acidosis
Alkalosis: Abnormally high blood pH
Acidosis: Abnormally low blood pH
Buffer Control of Blood pH
Types of buffer systems
Bicarbonate buffer - Consists of a mixture of bicarbonate ions (HCO 3−) and carbonic acid (H2CO 3)
regulated by the kidneys and the respiratory system
Phosphate buffer
Plasma proteins
Buffers are resistant to pH changes because they have an acid and a base part
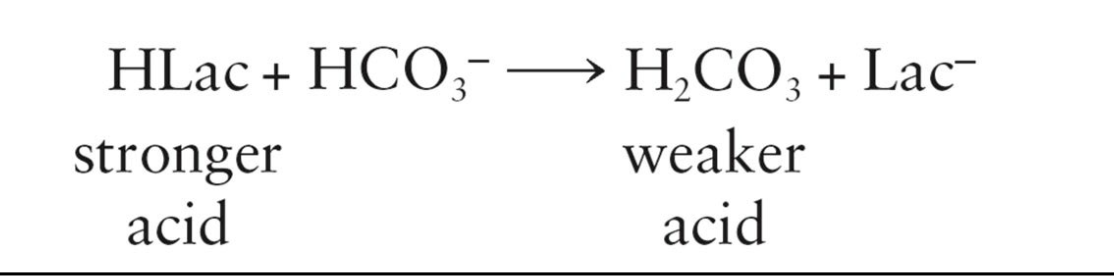
Respiratory Control of Blood pH
Respiratory system helps control the acidity of blood by regulating the elimination of CO2 and H2O
When more CO2 and H2O are exhaled, more carbonic acid is removed from the blood
Blood pH is raised to a more alkaline/basic level
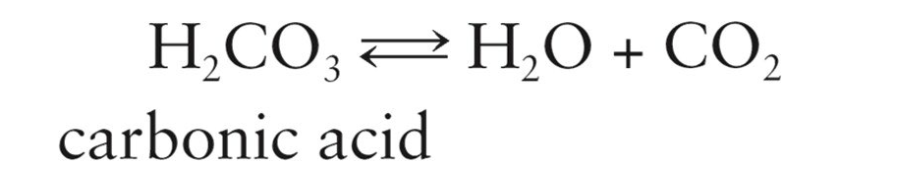
Hyperventilation
Rapid, deep breathing that is caused by an increase in the CO2 of arterial blood or a decrease in the arterial pH level below 7.38
Hypoventilation
Slow, shallow breathing
Less CO2 is exhaled, and higher concentration of carbonic acid remaining in the blood lowers the pH back to normal
Urinary Control of Blood pH:
Reactions involved in the excretion of H+ ions by the kidneys
breathing in CO2 gives us a lot of bicarbonate ions, which dissociates rapidly
Hydrogen ions diffuse into the developing urine
A Na+ ion passes into the tubule cells for every H+ ion that enters the urine
Decrease in CO2 and increase in HCO3− increases the blood pH levels back to normal
Developing urine picks up the hydrogen ions, which react with
buffering ions present in the urine
Presence of phosphate buffer system
Respiratory Acid–Base Imbalances
Respiratory acidosis and alkalosis
Result from abnormal breathing patterns
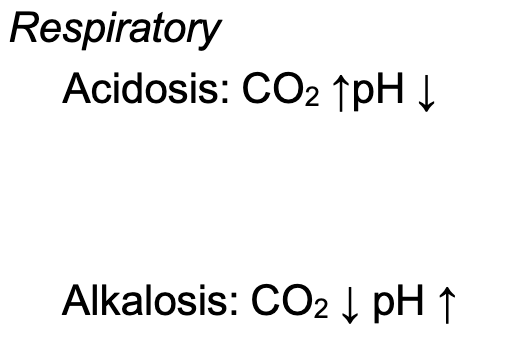
Respiratory Alkalosis
Caused by hyperventilation that increases loss of CO2
Increases blood pH
Treatment
Rebreathing one's own exhaled air by breathing into a paper bag
Administering CO2
Treating the causes of hyperventilation
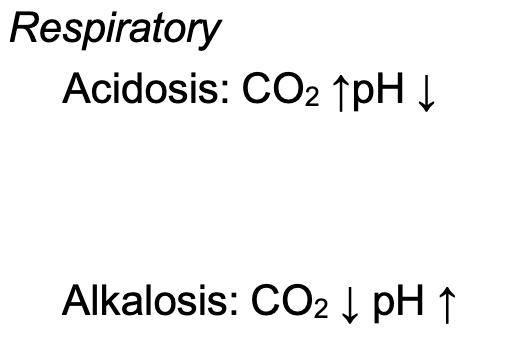
Respiratory Acidosis
Result of hypoventilation that decreases the loss of CO2
Decreases blood pH
Causes:
Overdose of narcotics or barbiturates
Anesthesia
Lung disease, such as emphysema and pneumonia
Object lodged in the windpipe
Treatments:
Identifying the underlying cause
IV administration of isotonic sodium bicarbonate solution or hemodialysis
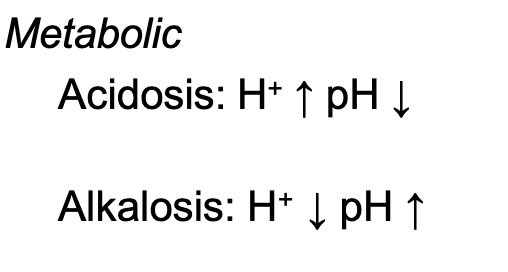
Metabolic Acidosis
Caused by factors other than abnormal breathing
Serious problem in diabetes mellitus
Occurs temporarily after heavy exercise
Caused by severe diarrhea and aspirin overdose
Triggered by the increase in H + that leads to lower blood pH
Symptoms and Treatment of Metabolic Acidosis
Symptoms:
Hyperventilation
Increased urine formation
Thirst
Drowsiness
Headache
Disorientation
Treatment:
Insulin therapy
Intravenous bicarbonate
Hemodialysis
Metabolic Alkalosis
Caused by factors other than abnormal breathing
Body loses acid because of prolonged vomiting or ingestion of alkaline substances
Decrease in H + in bodily fluids leads to an increase in blood
pH
Symptoms
Hypoventilation
Numbness
Tingling
Headache
Causes:
Kidney disease, prolonged vomiting, excessive intake of baking soda