T cells
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
The cells from the immune system derive from precursors in the bone marrow
Pluripotent hematopoietic stem cell → common myeloid and common lymphoid progenitor cells → the common lymphoid progenitor gives rise to T cells
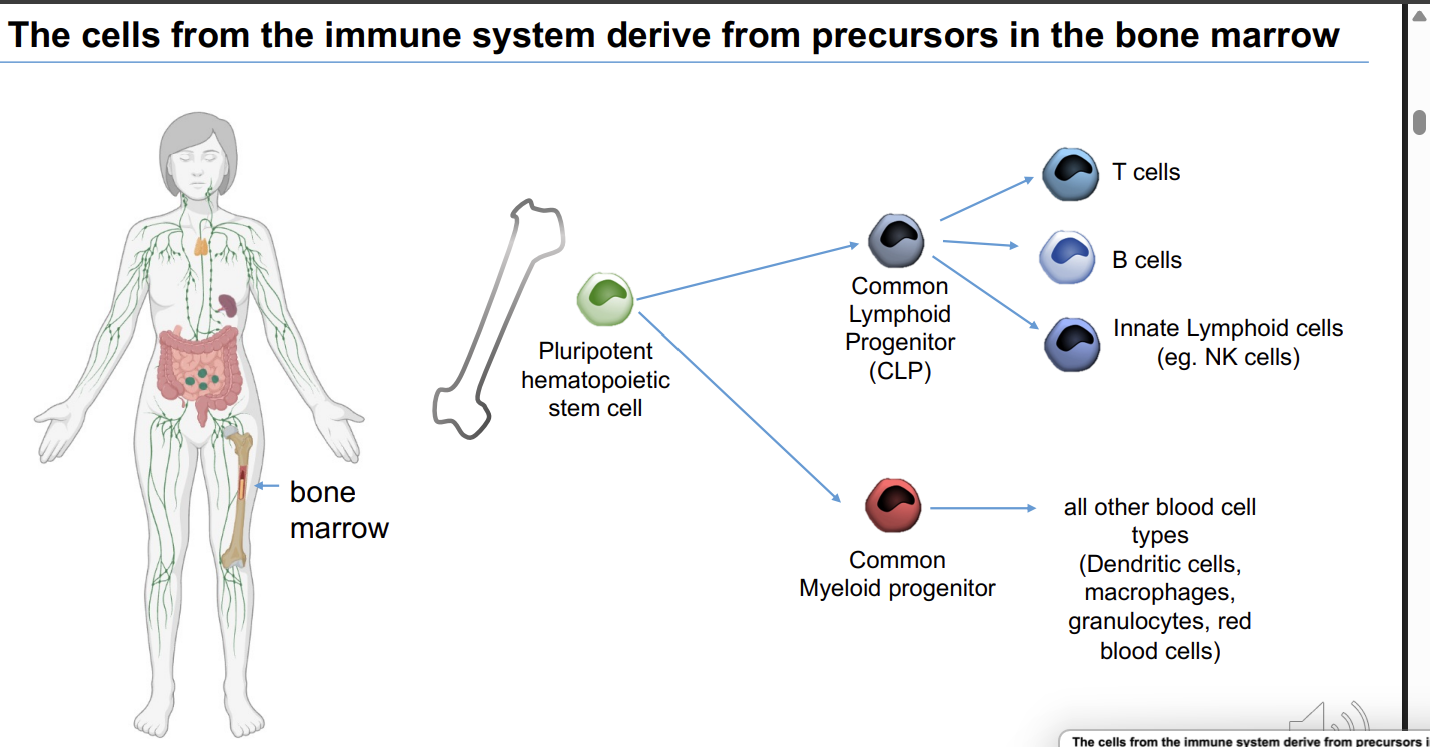
what does the common lymphoid progenitor give rise to? 3
T cells
B cells - made and fully mature in the bone marrow
Innate lymphoid cells - eg NK cells
what does Common Myeloid progenitor give rise to?
all other blood cell types (Dendritic cells, macrophages, granulocytes, red blood cells)
where do T cell precursors originate and migrate to?
T cell precursors originate in the bone marrow
T cell precursors migrate to the thymus where they maturate.
what are some examples of T cells? 3
Cytotoxic T cells (CTL)
T helper (Th) cells →Th1, Th2,Th17
Regulatory T cells (Tregs)
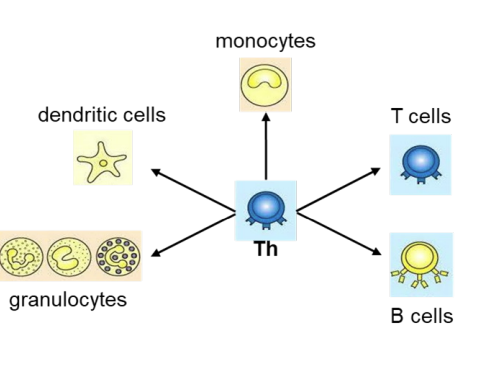
what do helper T cells do?
Helper T cells (Th cells) coordinate the adaptive immune response including activation of immune cells (T cells, B cells, granulocytes, monocytes→macrophages and can activate dendritic cells). and can recruit granulocytes, macrophages and dendritic cells to the site of infection
Activation of immune cells is mediated by cytokines released by Th cells.
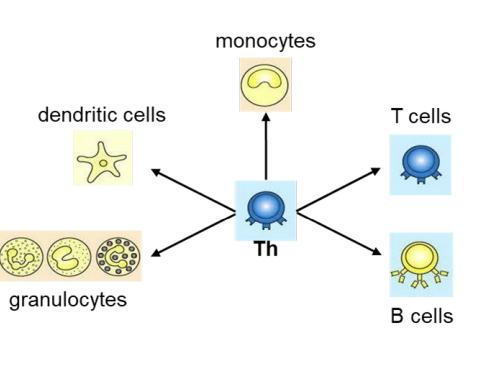
Activation of immune cells is mediated by
cytokines released by Th cells.
Cytokines
secreted proteins including growth factors, differentiation factors as well as chemo attractants.
bind to specific receptors on surface of target cells and initiate cell signalling (hormone-like).
Interleukins
20 chemokines (chemoattractant cytokines), are named by their N-terminal cysteine amino acid sequence, e.g. CC, CXC, followed by an L (for ligand) while their receptors have an R e.g CCR
Other cytokines: Interferons (IFN)-α, β, γ; Tumour Necrosis Factor (TNF)-α, Transforming Growth Factor (TGF)-β, etc…
what are interleukins really important for?
(IL) –are important for ”tuning” the immune responseIL-1, IL-2
….IL-41 (numbered in order of discovery)
what are 5 examples of what cytokines can be?
secreted proteins including growth factors, differentiation factors as well as chemoattractants (eg cytokines).
interleukins
interferons
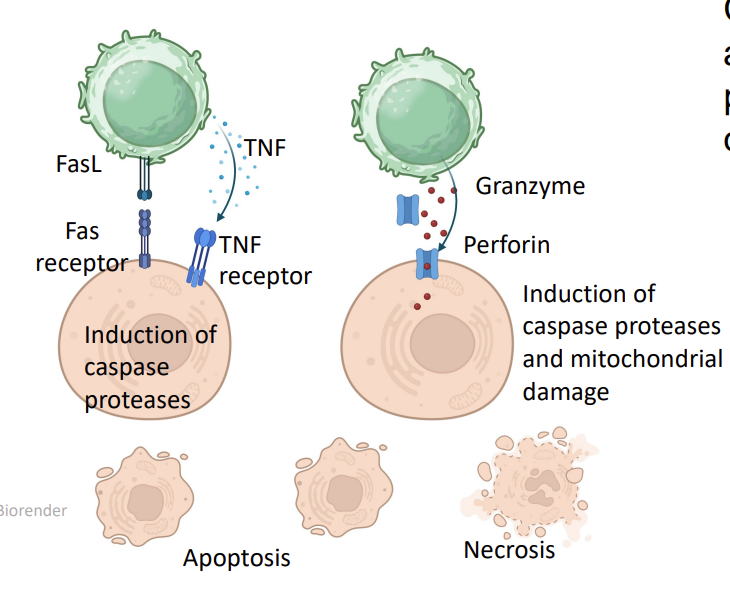
Cytotoxic T lymphocyte (CTL)
CTLs recognize and kill cells that are infected by intracellular pathogens (viruses, some bacteria) or are transformed (cancer cells)
this leads to secretion of cytotoxic cytokines e.g. Tumour Necrosis Factor (TNF)- induction of caspase proteases → apoptosis
Fas ligand (TNF homologue) binds to Fas (TNF receptor homologue)
CTL can also secrete perforin and granzymes →necrosis
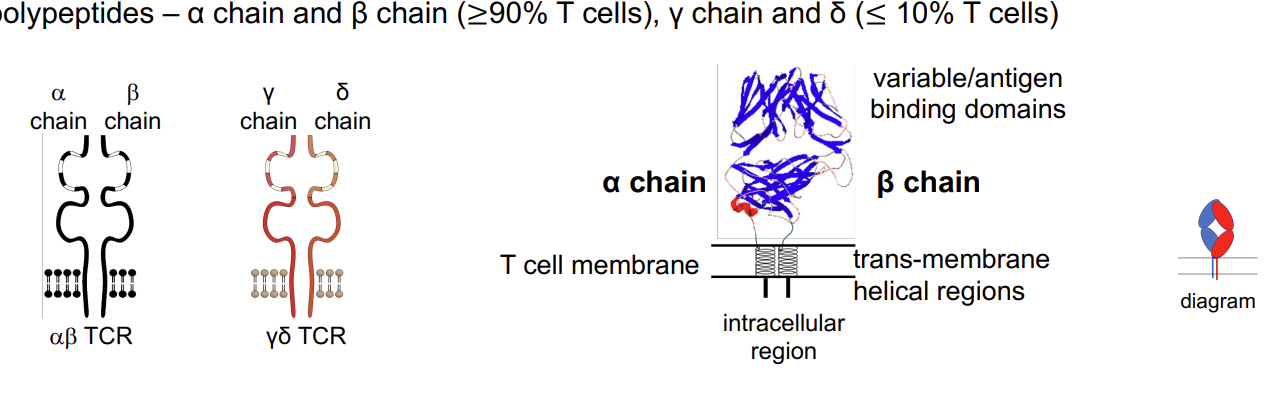
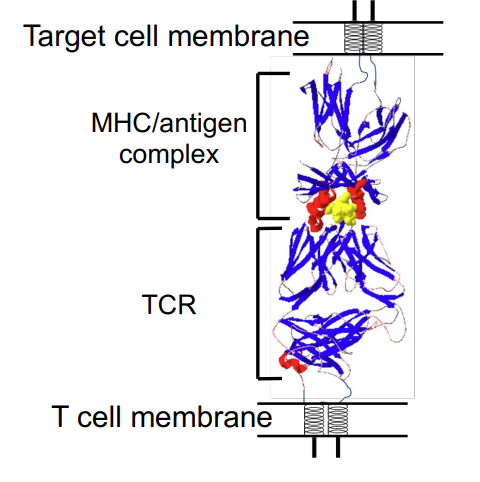
T cell: Antigen recognition (i)
All T cells express a cell surface (membrane bound) antigen receptor (TCR) formed by 2polypeptides – α chain and β chain (≥90% T cells), γ chain and δ (≤ 10% T cells)
Each T cell has receptor of unique specificity.
TCR does not bind antigen directly
TCR does not bind antigen directly - unlike B cell receptor, it cannot recognise natural antigens (cannot identify a random bacteria) but it can identify a fragment that is presented on an MHC molecule expressed on the surface of the target cell → allows the T cell to come into very close contact with it’s target cell/APC
TCR does not bind antigen directly…what is the condition for it binding?
it will only bind to fragments of antigens (peptides) that are bound to major histocompatibility complex (MHC) proteins on the surface of the target cell. Peptides are produced by intracellular proteases that degrade antigens of infectious microroganisms.
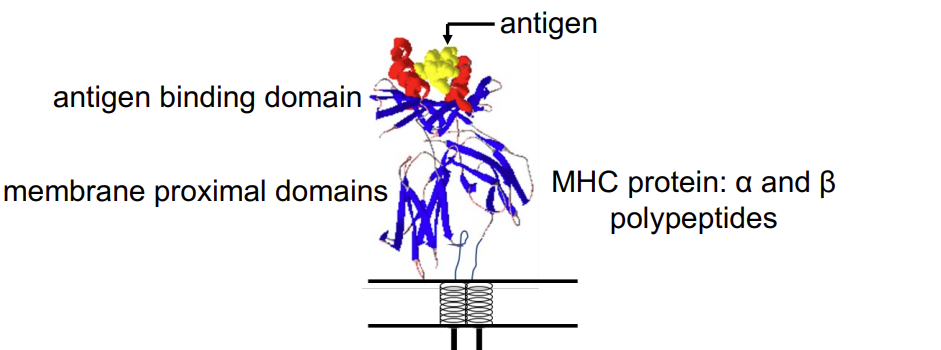
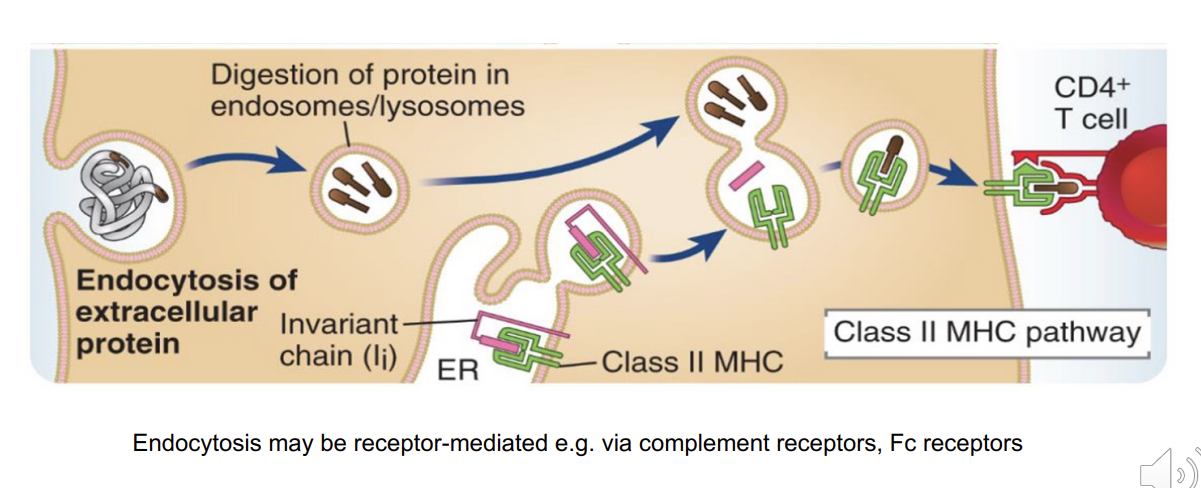
Structure of MHC class II with bound peptide
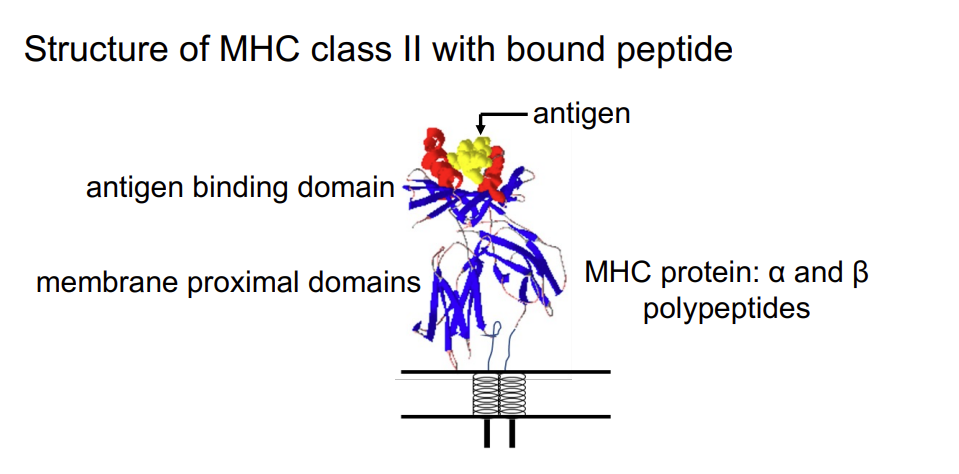
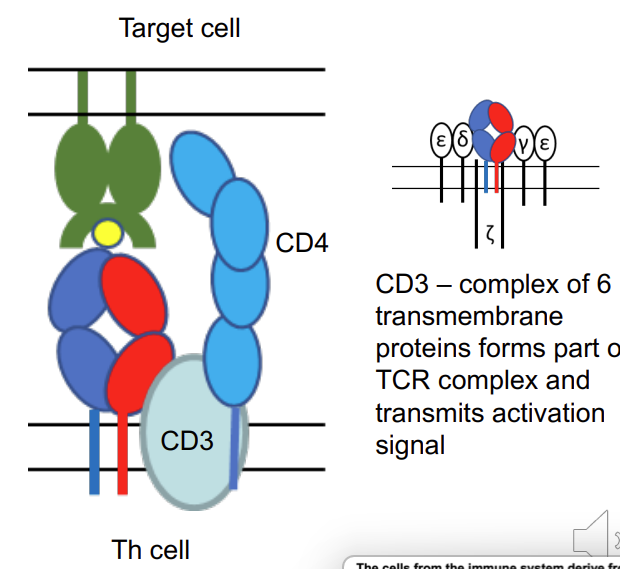
what is CD3?
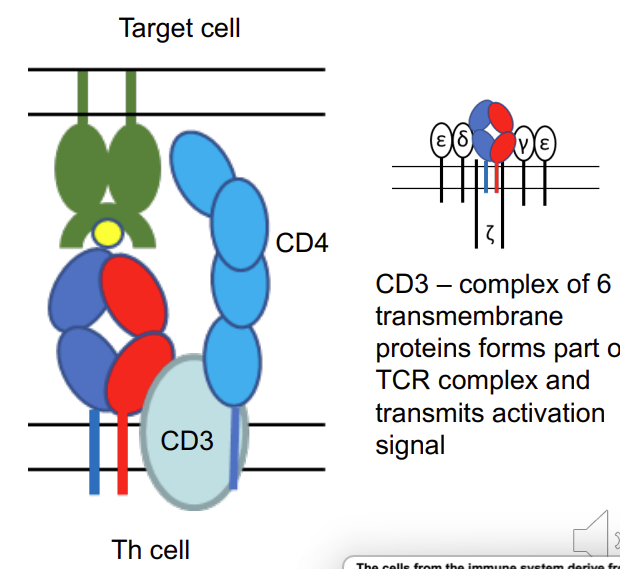
→ CD3 – complex of 6 transmembrane proteins forms part of TCR complex and transmits activation signal
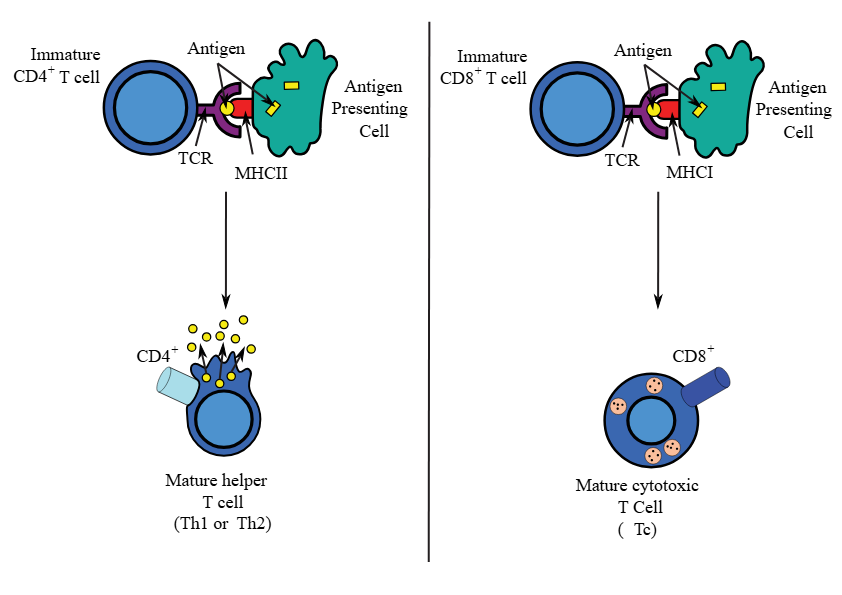
MHC class II
T helper cell binds to antigen in conjunction with the MHC II (and CD4 T cell)
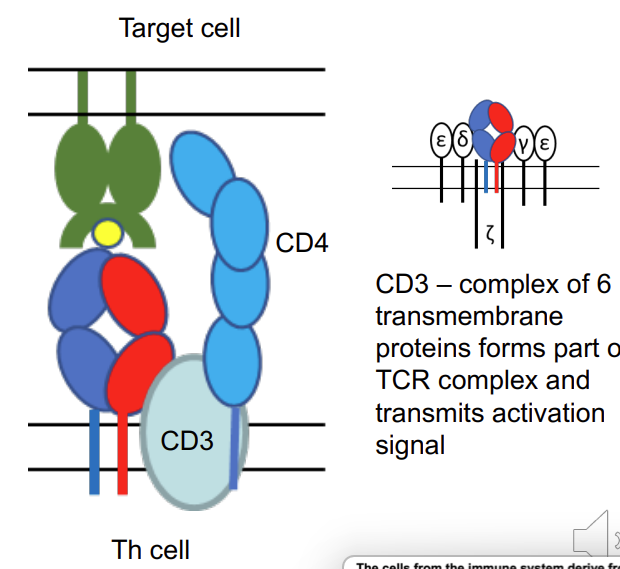
what is happening in this image?
T helper cell binds via TCR to MHC II complex with peptide. TCR of Th is associated with CD4 which binds to MHC II -CD4 is an accessory protein - stabilises the reaction between the T cell receptor and the MHC
MHC class 1 vs class 2
MHC class I: overall structure similar but α polypeptide is larger and forms complete peptide binding domain. β2microglobulin (β2m) not transmembrane - still there just not transmembrane .
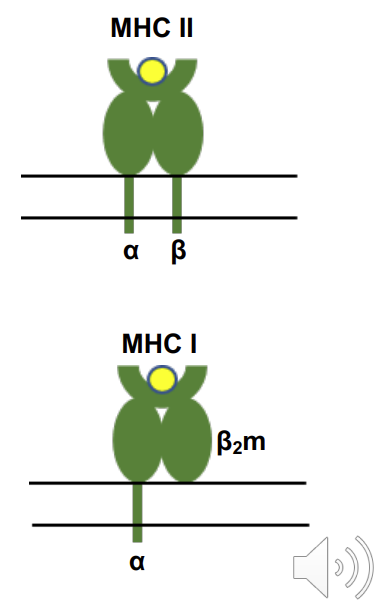
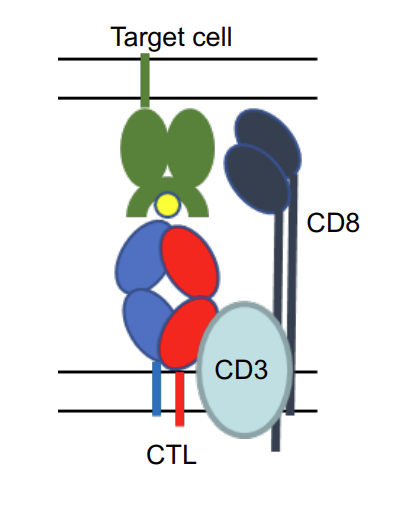
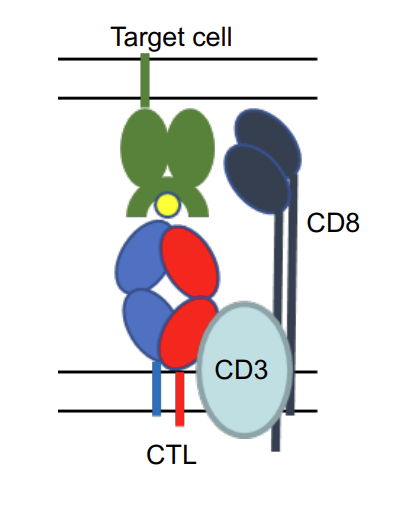
what type of MHC is this?
CTL binds to MHC I complex with peptide. TCR of CTL is associated with CD8 which binds to MHC I.
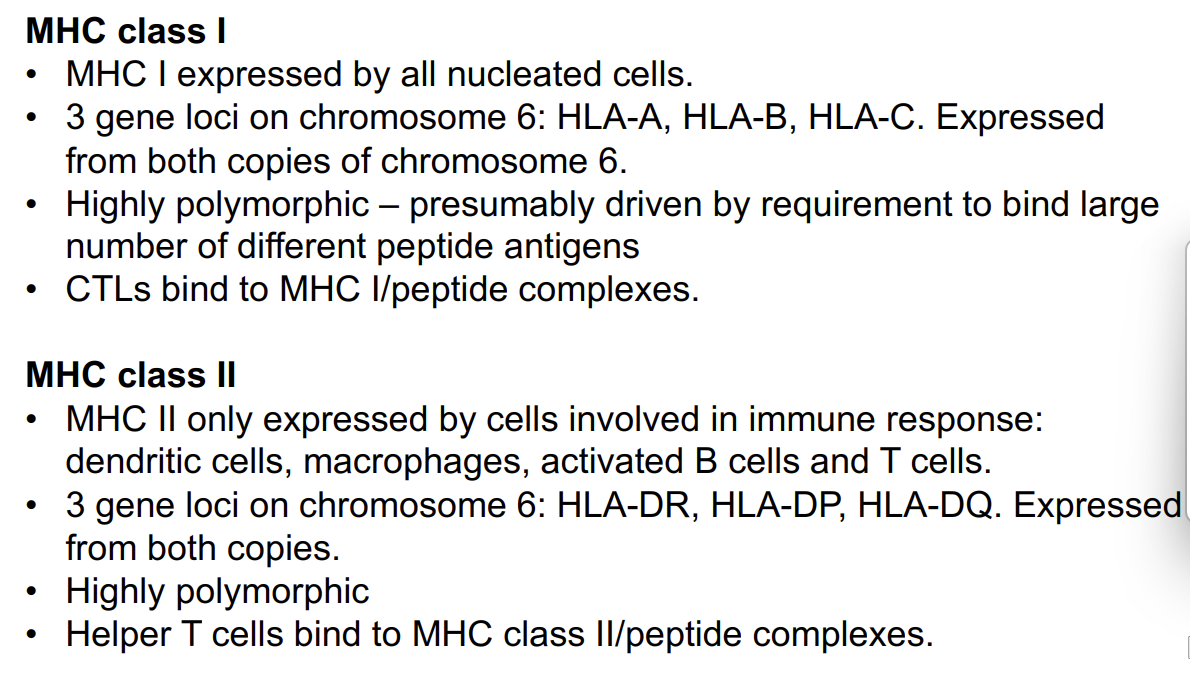
MHC class I - the facts
MHC I expressed by all nucleated cells - everything except RBCs or platelets
3 gene loci on chromosome 6: HLA-A, HLA-B, HLA-C. Expressed from both copies of chromosome 6.
Highly polymorphic – presumably driven by requirement to bind large number of different peptide antigens
CTLs- cytotoxic T lymphocytes bind to MHC I/peptide complexes.
MHC class II - the facts
MHC II only expressed by cells involved in immune response: dendritic cells, macrophages, activated B cells and T cells.
3 gene loci on chromosome 6: HLA-DR, HLA-DP, HLA-DQ. Expressed from both copies.
Highly polymorphic
Helper T cells bind to MHC class II/peptide complexes.
what cells express MHC I vs MHC class II?
what is antigen processing?
T cells expressing αβ T cell receptor recognise peptides that are complexed with MHC molecules aka they have an MHC class II receptor along with a CD4 receptor.
Antigenic peptides presented by MHC class I are derived from intracellular pathogens that synthesise or release proteins in the cytosol. These are recognised by CTLs.
Antigenic peptides presented by MHC class II are derived from proteins of extracellular pathogens that are endocytosed by antigen presenting cells.
These are recognised by Helper T cells.
The MHC I and MHC II pathways are in separate cellular compartments.
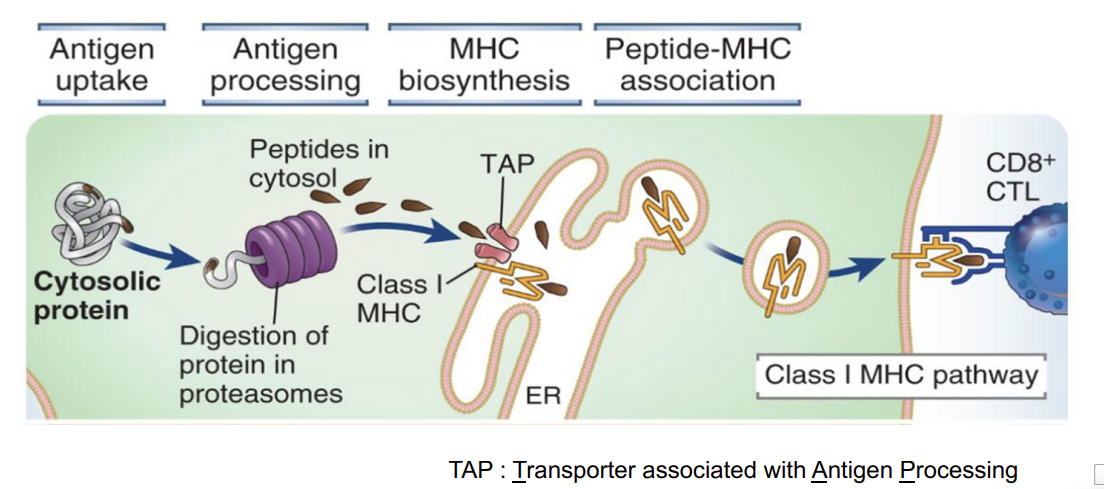
MHC I antigen processing pathway (any nucleated cell type)
the antigen is found within the cytosol - eg from a virus
these proteins can be targeted for breakdown within the proteosome
the breakdown of the protein within the proteosome generates lots of small peptides
these can be transported by a transporter called TAP into the lumen of the endoplasmic reticulum
they then bind to class I MHC molecules
once bound and then stabilised - transported to the cell surface and is now presented to CD8 T cells
this allows a T cell to basicially ‘see’ what is happening inside the cell
MHC II antigen processing pathway (antigen presenting cells (APC) only i.e. dendritic cells, macrophages, B cells)
endocytosed
digested within endosomes/lysosomes
MHC class II molecules resides in the endoplasmic reticulum - but is bound to an invariant chain which occupies the binding groove
once the MHC II transferred into the lysosome vesicle → invariant chain is exchanged for the antigenic peptide which can then bind to the binding groove
MHC class II is then transported to the cell surface
can now be presented to CD4 T cells
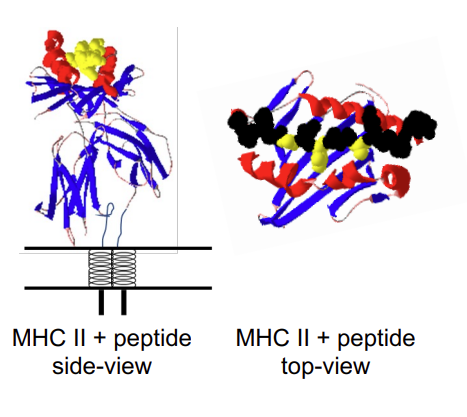
MHC peptide binding is highly promiscuous
Few residues in peptide critical for binding.
MHC I binds peptides of 8-10 residues, only 1 or 2 contribute to binding.
MHC II binds peptides 12-17 residues, only 2 or 3 contribute to binding.
All other positions can vary so very large number of peptides can bind to any MHC molecule.
In this example, 16 amino acid peptide, 3 positions (yellow) required to bind MHC.
antigen is nestled in the binding groove of the MHC molecule
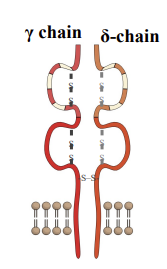
T cell recognition of other antigenic molecules
T cells that express γẟ(gamma delta) T cell receptor
derived from separate gene loci with fewer V gene segments
reflect reduced diversity of TCR specificity
γẟ T cell recognition of bacterial-derived metabolites or lipids associated with MHC class I-like surface receptors confirmed in several studies
Naïve T lymphocytes are activated by
specialised antigen presenting cells (APCs):generally dendritic cells (DC), macrophages, B cells.
this is because an activated T cell response can potentially be very dangerous for the body - may kill own cells - ie autoimmune disease
only specific cells can activate a T cell response

APCs
APCs express both MHC class I and MHC class II proteins so bind to and activate both CD4 Th and CD8 Tc lymphocytes.
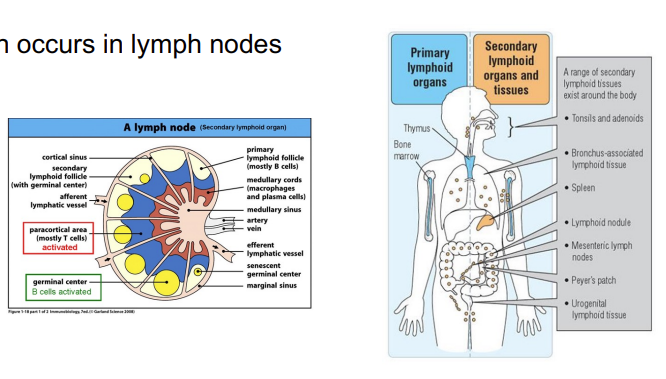
activation occurs in the lymph nodes → the number of APCs is very high - maximises the chances that the T cell that has the right combination of MHC and peptide so that the T cell can be activated
cells that respond to an intracellular infection display the protein via MHC class 1 - these are not considered professional APCs
since professional APCs are nucleated, they express both MHC class 1 and 2
where does activation of T cells occur? Th activation requires 3 signals:
MHC-II:antigen → TCR +CD4, CD3
Co-stimulatory proteins, CD80/CD86 → CD28 (1+2 induce IL-2 secretion)
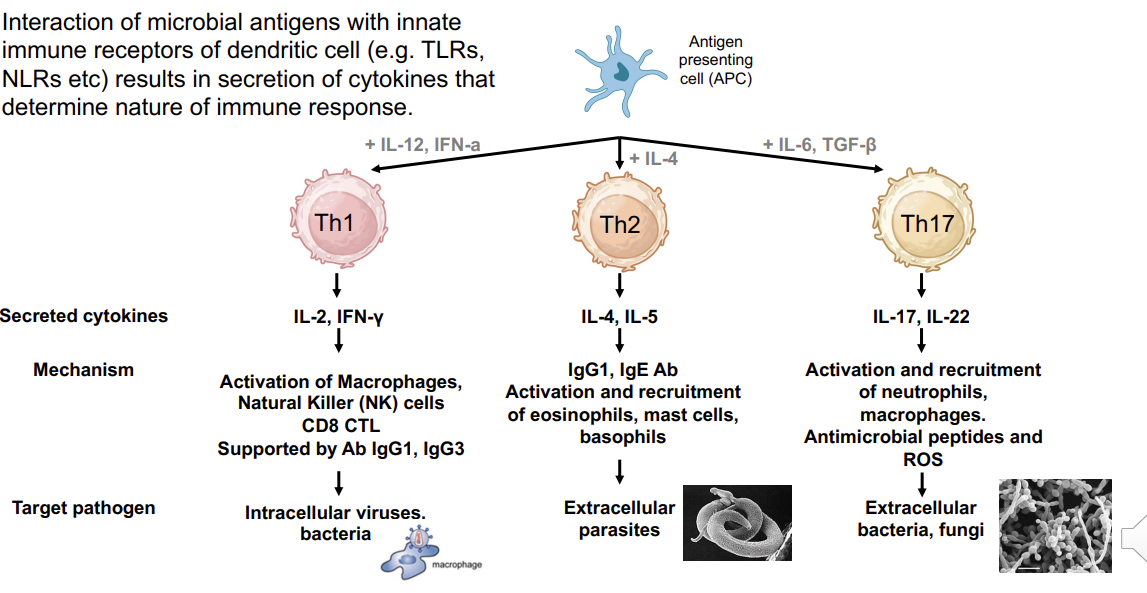
Cytokines secreted from DC (APC) determine type of Th response
Induction of CD40L transient expression on Th cell signals to APC (via CD40) to become fully activated/licensed → can now also activate cytotoxic T cells
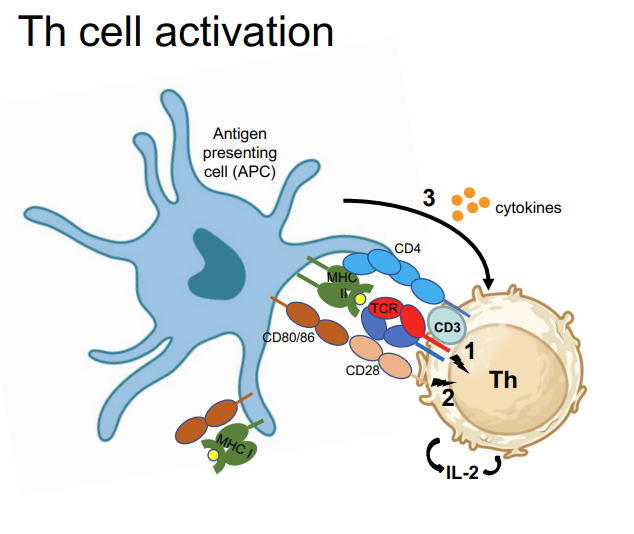
T cells that have only received signal 1 (see previous slide) will be regarded as anergic
cannot respond to antigen again - safeguarding mechanism
licensed APCs
Induction of CD40L transient expression on Th cell signals to APC (via CD40) to become fully activated/licensed → can now also activate cytotoxic T cells
Activated Th cells proliferate giving rise to
effector and memory Th cells
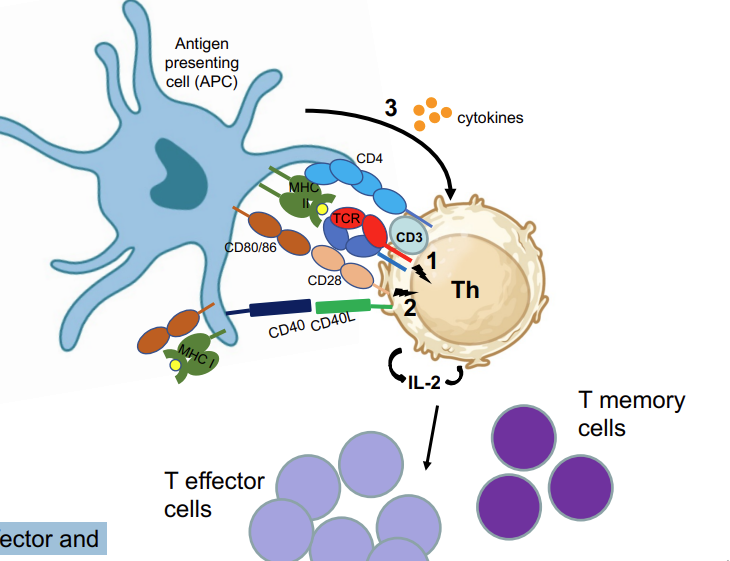

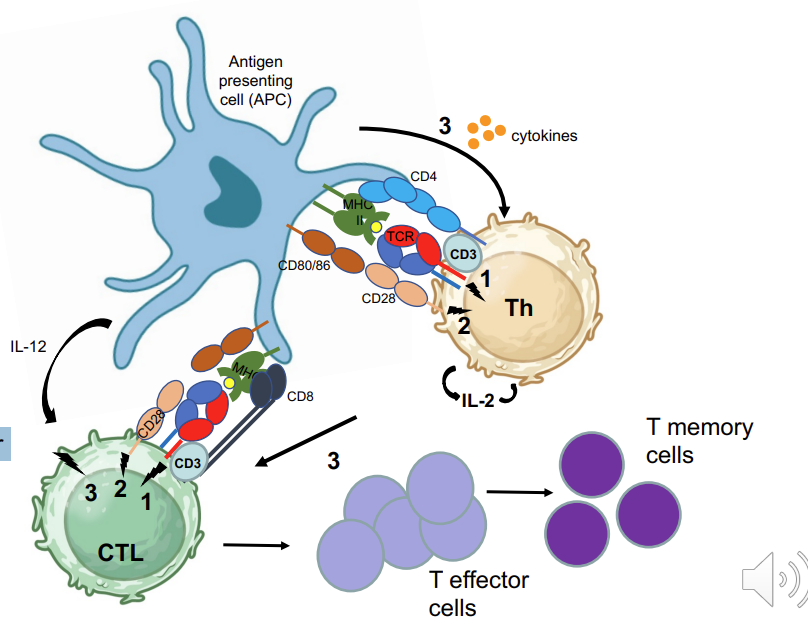
CD8 Cytotoxic T cell activation 4
Tc activation is complex:
MHC-I:antigen TCR, CD3
Co-stimulatory proteins, CD80/CD86 → CD28
Requires Th activity via cytokines → production of IL-2 →stimulation via APC derived inflammatory cytokines such as IL-12
Activated CTL proliferate: effector and memory
Cytokines (signal 3) secreted by activated dendritic cell or other antigen presenting cell determines type of Th cell response
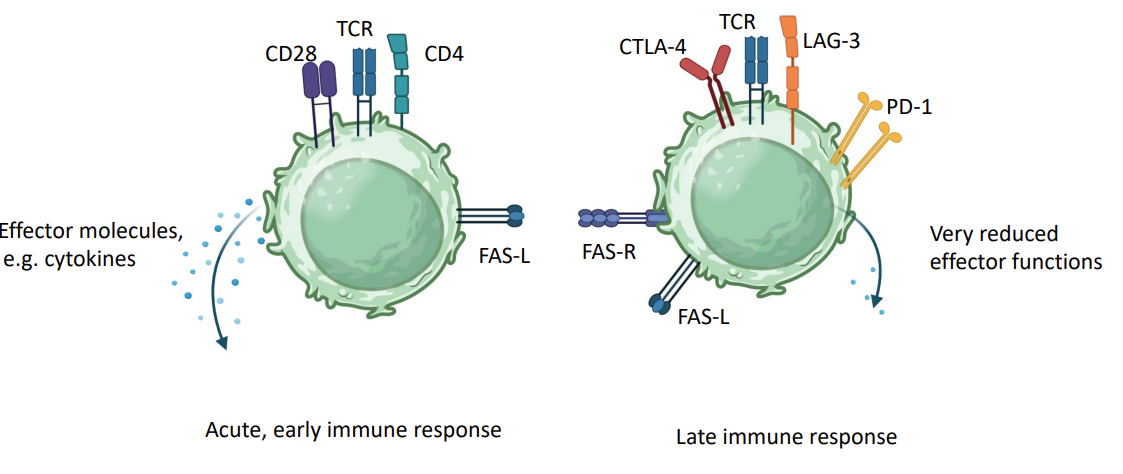
Switching off the immune response
As immune response matures, stimulating receptors (CD28, CD4) replaced with inhibitory receptors (CTLA-4, LAG3).
T cells expression of apoptotic receptors e.g. FAS - fratricide - they kill each other
this leads to the reduction of immune cells we have
part 3 - T cell selection in the thymus
T cells can recognise a huge range of antigens, however each T cell recognises a single/limited number of antigens
gene rearrangement and mutagenesis to give the range of diversity needed in T cell receptors
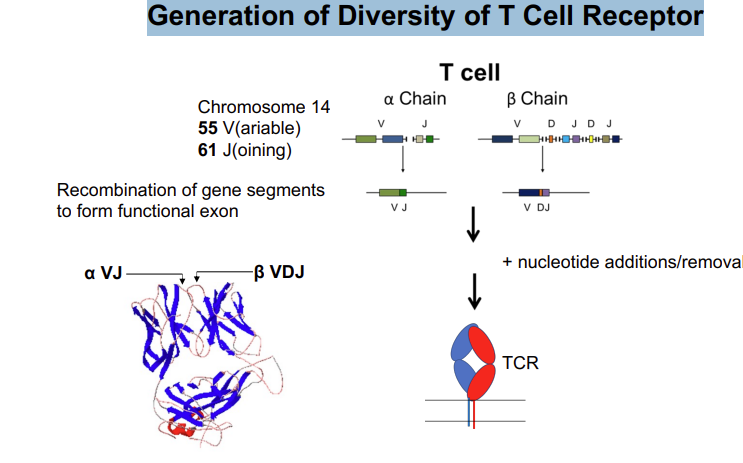
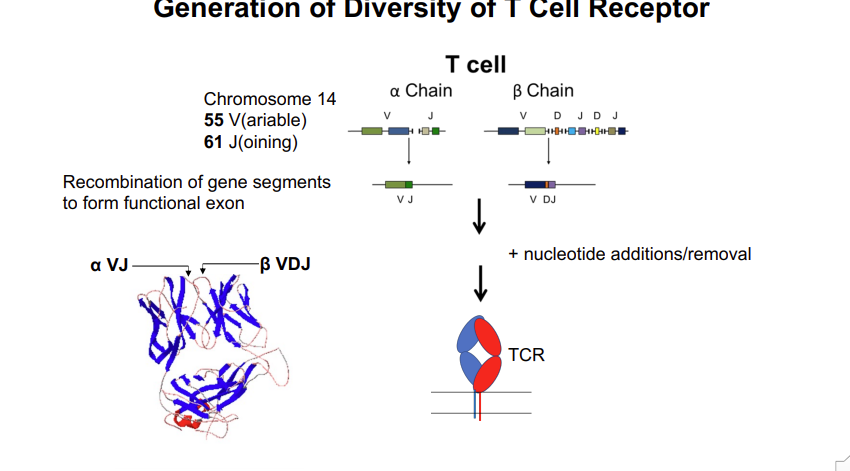
Generation of Diversity of T Cell Receptor - gene rearrangement of the variable antigen binding region
the locus of the T cell receptor alpha chain
55 V regions and 61 joining regions → one of each needs to be brought together to form a functional exon
this requires the rearrangement of the chromosome at this locus - which is mediated by the enzymes rag 1 and 2
looping of DNA randomly aligns one of the V and J segments → religated to form a continuous exon
inclusion/removal of nucleotides → adds further diversity
3500 different exons for alpha chains
B chain has fewer J segments but 2 more D segments 1500 different antigen binding regions
any of the alpha chains can bind with any of the beta chains → adds further diversity 2×107
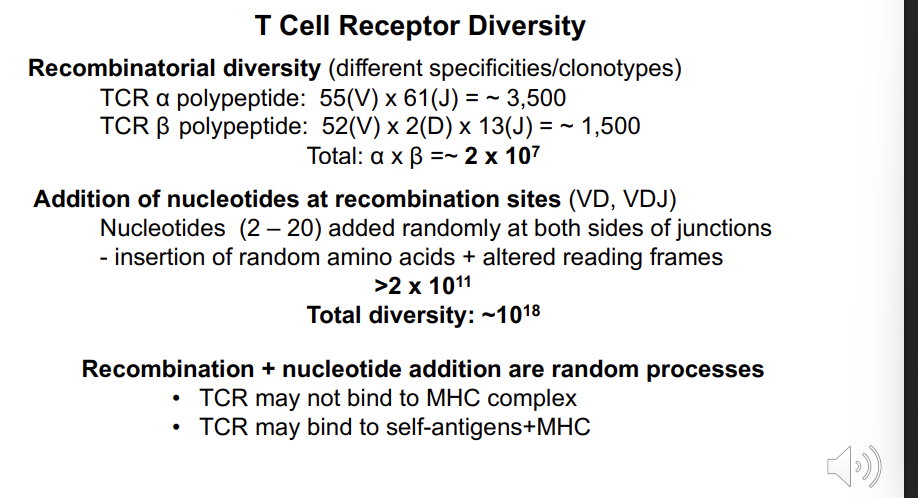
Total: α x β =~ 2 x 107 - how is this increased further?
Addition of nucleotides at recombination sites (VD, VDJ) Nucleotides (2 – 20) added randomly at both sides of junctions - insertion of random amino acids + altered reading frames >2 x 1011
Total diversity: ~1018
Recombination + nucleotide addition are random processes
TCR may not bind to MHC complex
TCR may bind to self-antigens+MHC
these scenarios should be avoided
Positive Selection of T Cells in Thymus Cortex - overview
Immature T cells express TCR as well as CD4 and CD8 (double positive, DP)
Cortex
T cells that do not bind self MHC molecules undergo apoptosis
T cells that bind to self MHC II (on epithelial cells or dendritic cells) in thymus cortex become CD4 single positive (SP) cells
T cells that bind to MHC I become CD8 SP cells
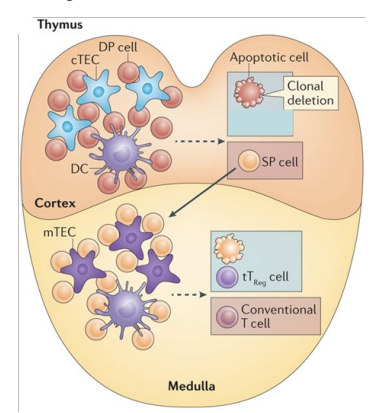
Positive Selection in Thymus Cortex - fate of the double positives
cortical endothelial cell in the thymus
expresses both MHC class 1 and 2 molecules
double positive T cell receptor comes - cannot bind to MHC 1 OR 2 → apoptosis → 90%
binds MHC 2 → becomes CD4 → moves to medulla
binds MHC 1 → becomes CD8 → moves to the medulla
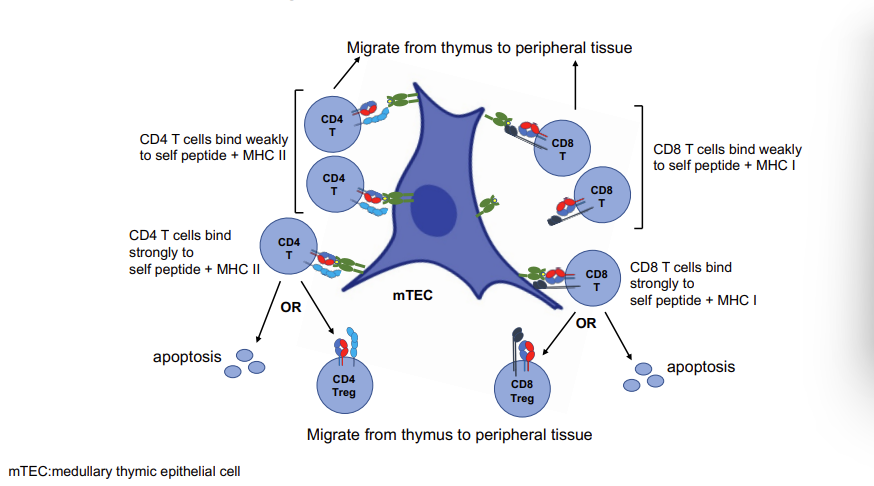
Negative Selection of T Cells in Thymus - medulla
because they can recognise MHC → wave 2 of selection for CD4 and CD8 cells
this is called negative selection and is important to exclude T cells which have a VERY strong attraction to MHC molecules → which could cause autoimmune disease
Surviving SP T cells migrate to medulla
SP T cells that bind weakly/moderately to self antigens associated with MHC, survive
SP T cells that bind strongly to self antigens/MHC undergo apoptosis or become Treg cells.
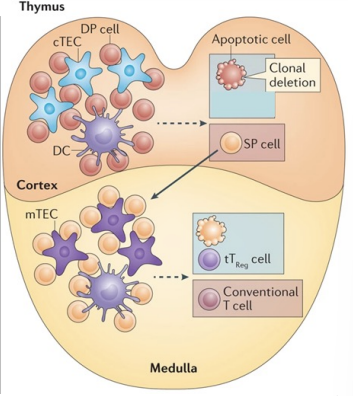
Negative Selection in Thymus Medulla
‘self peptides/MHC’ - thymic endothelial cells
crazy diversity - lots that are non functional or self reactive