avgeniii battery quiz
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
37 Terms
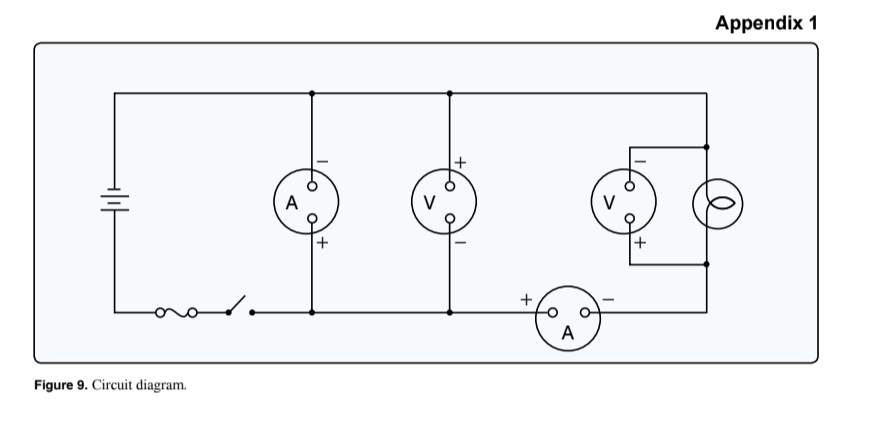
(Refer to Figure 9.) How many instruments (voltmeters and ammeters) are installed correctly?
Two
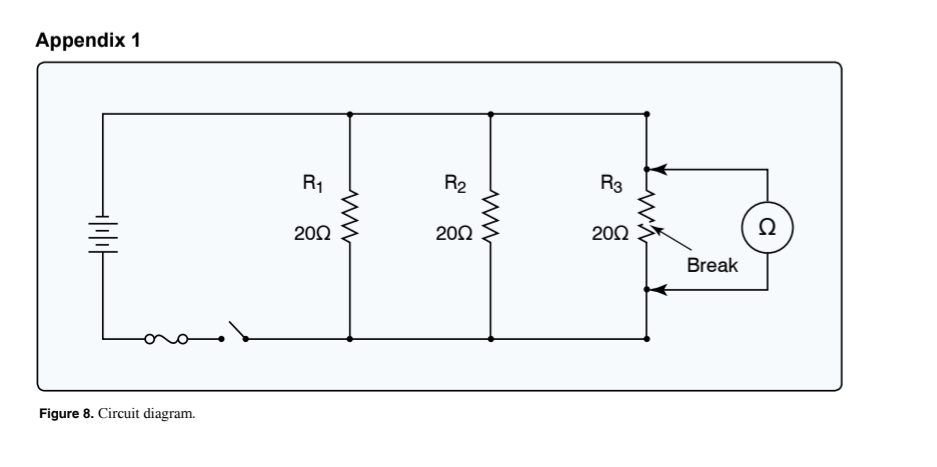
(Refer to Figure 8.) With an ohmmeter connected into the circuit as shown, what will the ohmmeter read?
10 ohms
The correct way to connect a test voltmeter in a circuit is
in parallel with a unit.
If each cell, connected in series, equals 2 volts, how would a 12-cell lead acid battery be rated?
24 Volts
In a P-N-P transistor application, the solid-state device is turned on when the
base is negative with respect to the emitter.
In an N-P-N transistor application, the solid-state device is turned on when the
base is positive with respect to the emitter.
Typical application for zener diodes is as
voltage regulators
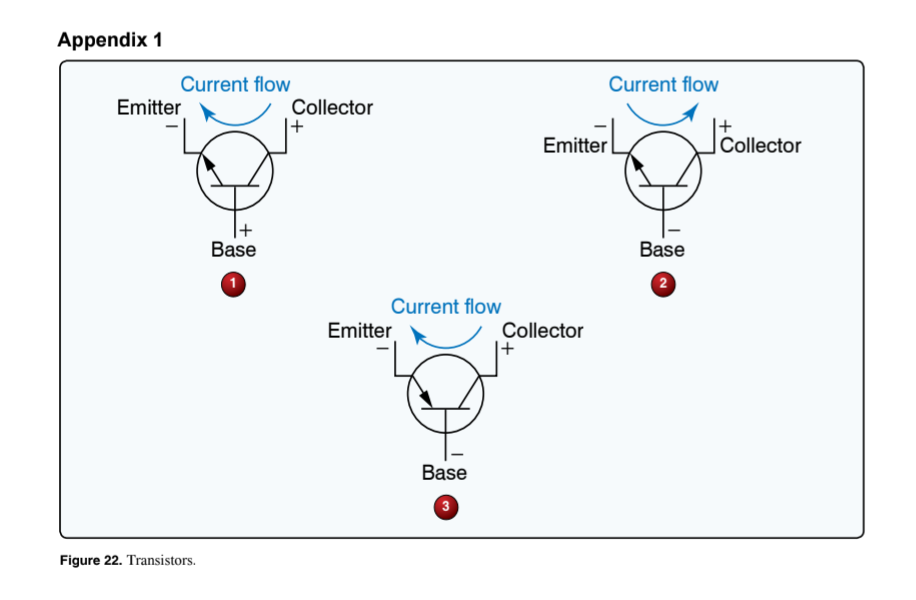
(Refer to Figure 22.) Which illustration is correct concerning bias application and current (positive charge) flow?
1
Forward biasing of a solid-state device will cause the device to
conduct
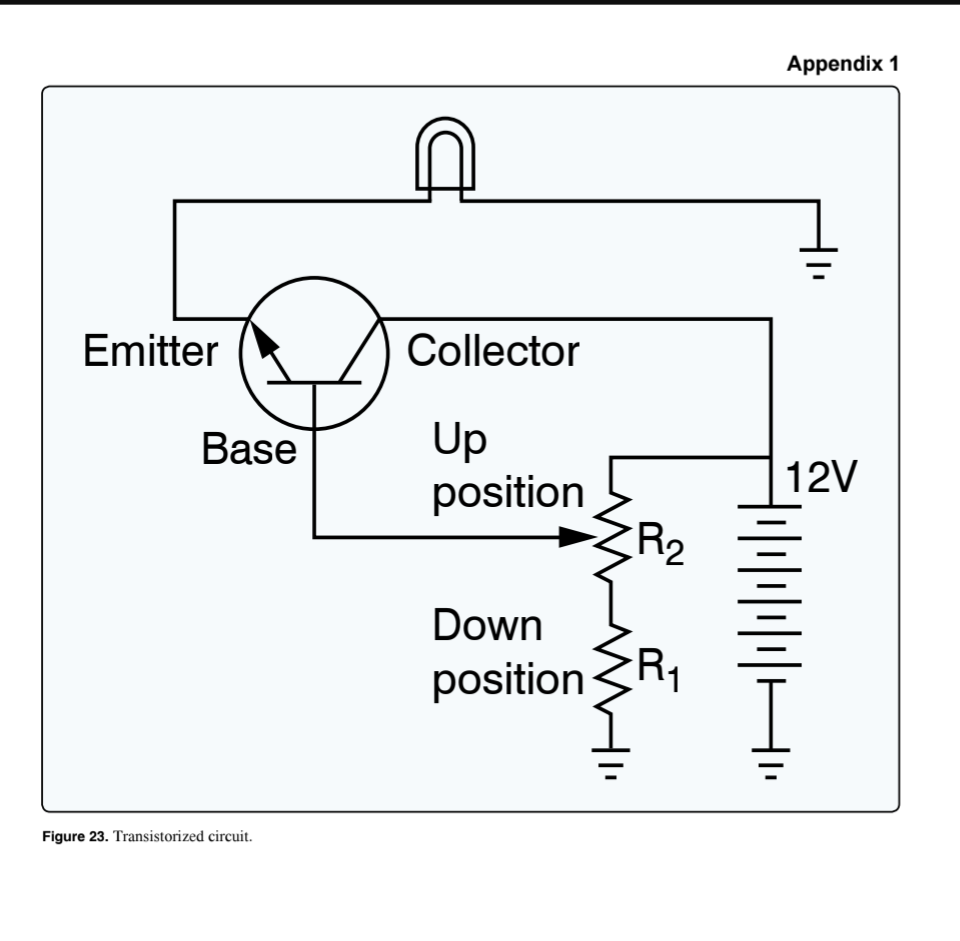
(Refer to Figure 23.) If an open occurs at R(1), the light
cannot be turned off
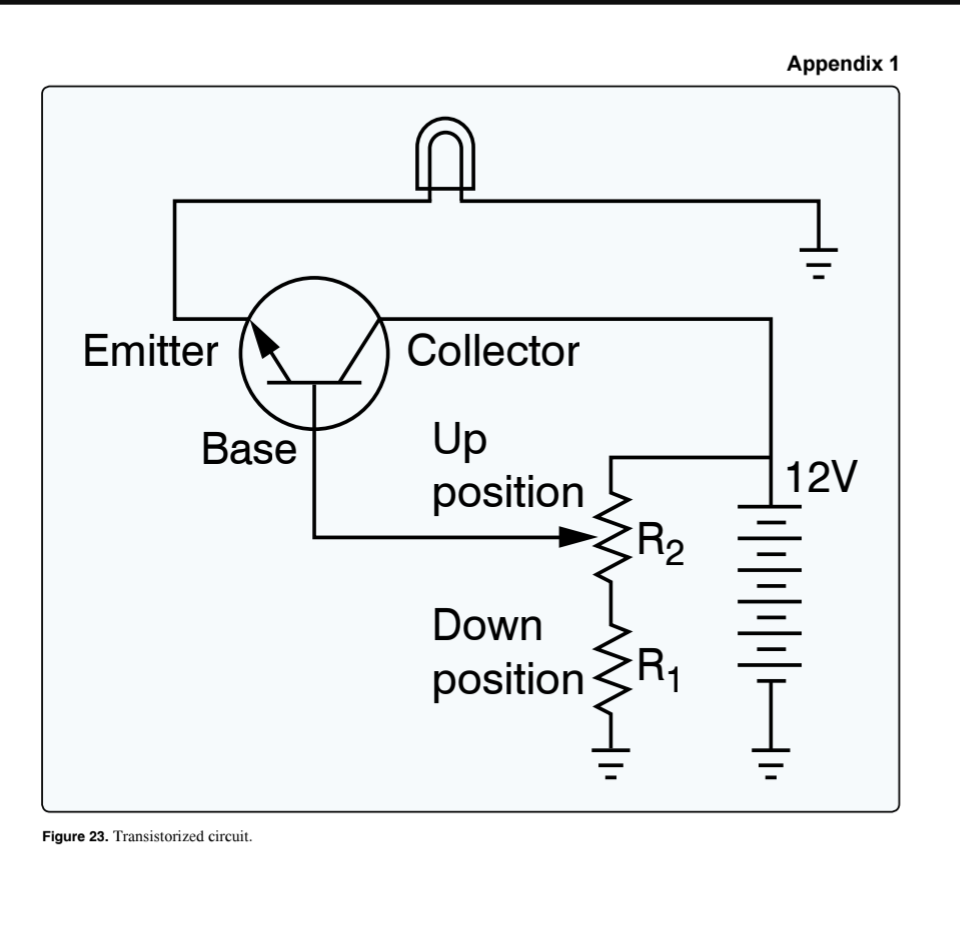
(Refer to Figure 23.) If R(2) sticks in the up position, the light will
be on full bright
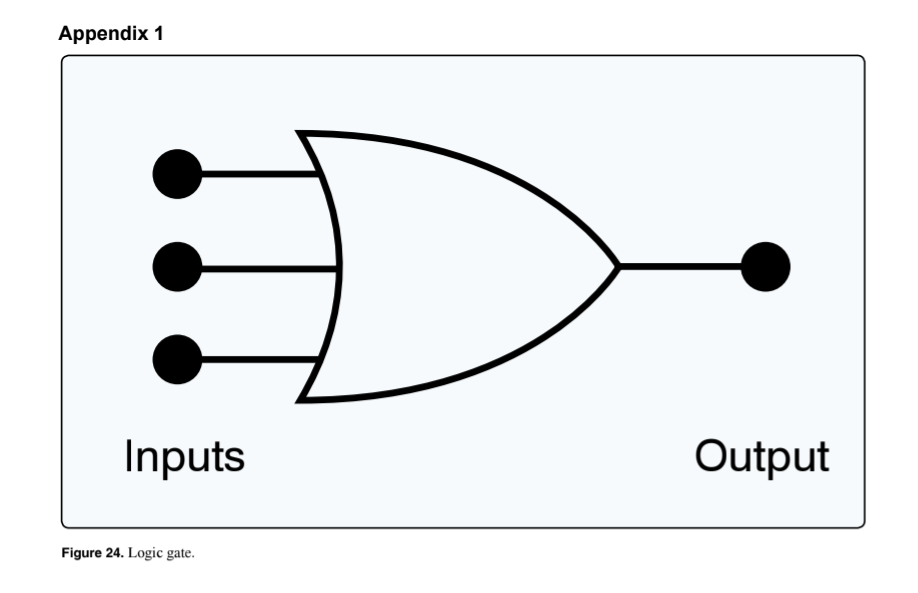
(Refer to Figure 24.) Which statement concerning the depicted logic gate is true?
Any input being 1 will produce a 1 output.
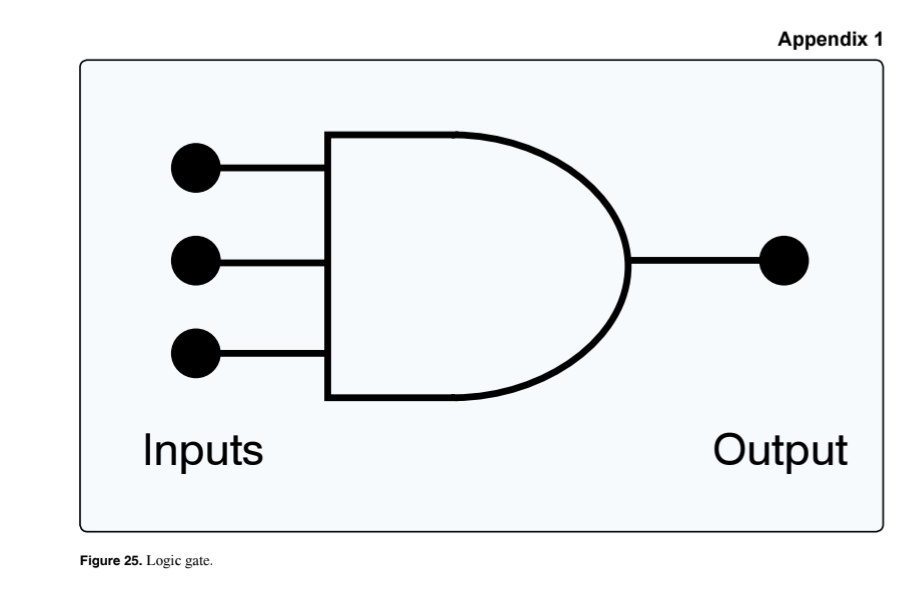
(Refer to Figure 25.) In a functional and operating circuit, the depicted logic gate's output will be 0
when one or more inputs are 0
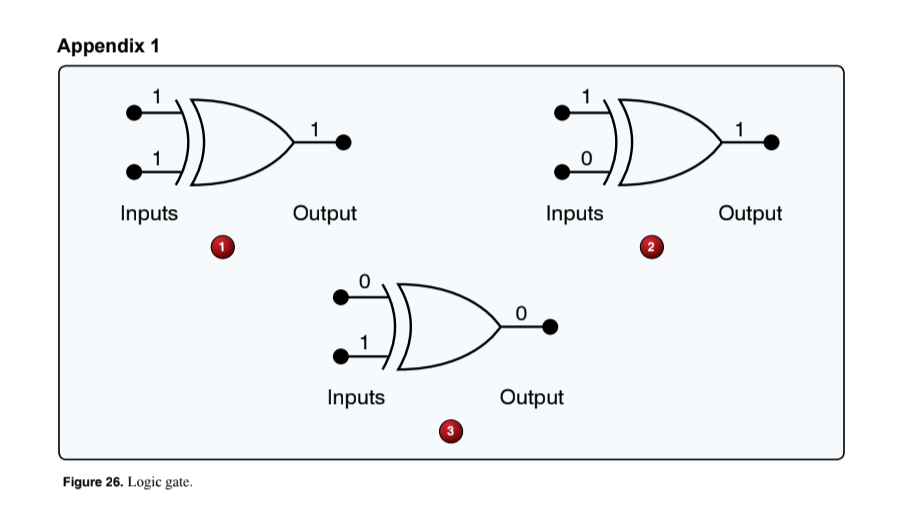
(Refer to Figure 26.) Which of the logic gate output conditions is correct with respect to the given inputs?
2
Which of the following logic gates will provide an active high out only when all inputs are different?
XOR
A lead-acid battery with 12 cells connected in series (no-load voltage = 2.1 volts per cell) furnishes 10 amperes to a load of 2-ohms resistance. The internal resistance of the battery in this instance is
0.52 ohm.
-If electrolyte from a lead-acid battery is spilled in the battery compartment, which procedure should be followed?
Apply sodium bicarbonate solution to the affected area followed by a water rinse
Which statement regarding the hydrometer reading of a lead-acid storage battery electrolyte is true?
The hydrometer reading does not require a temperature correction if the electrolyte temperature is 80°F.
A fully charged lead-acid battery will not freeze until extremely low temperatures are reached because
most of the acid is in the solution.
What determines the amount of current which will flow through a battery while it is being charged by a constant voltage source?
The state-of-charge of the battery.
Which of the following statements is false regarding the charging of several aircraft batteries together?
Batteries of the same voltage and same ampere-hour capacity must be connected in series with each other across the charger and charged using the constant-current method.
The method used to rapidly charge a nickel-cadmium battery utilizes
constant voltage and varying current.
The purpose of providing a space underneath the plates in a lead acid battery's cell container is to
prevent sediment buildup from contacting the plates and causing a short circuit.
Which condition is an indication of improperly torqued cell link connections of a nickel-cadmium battery?
Heat or burn marks on the hardware
The presence of any small amount of potassium carbonate deposits on the top of nickelcadmium battery cells in service is an indication of
normal operation.
What is the likely result, if any, of servicing and charging nickel-cadmium and leadacid batteries together in the same service area?
Contamination of both types of batteries would occur.
Which of the following best describes the operating principal in a nickel-cadmium battery installed in an aircraft?
To completely charge a nickel-cadmium battery, some gassing must take place; thus, some water will be used.
The electrolyte of a nickel-cadmium battery is the lowest when the battery is
in a discharged condition.
The electrolyte of a nickel cadmium battery is highest when the battery is
in a fully charged condition
The end-of-charge voltage of a 19-cell nickel-cadmium battery, measured while still on charge,
depends upon its temperature and the method used for charging
During discharge, nickel-cadmium batteries will show a lower liquid level than when at full charge because
the electrolyte becomes absorbed into the plates.
How can the state-of-charge of a nickel-cadmium battery be determined?
By a measured discharge
What may result if water is added to a nickel-cadmium battery when it is not fully charged?
Excessive spewing is likely to occur during the charging cycle.
In nickel-cadmium batteries, a rise in cell temperature
causes a decrease in internal resistance
Which of the following best describes the contributing factors to thermal runaway in a nickel-cadmium battery installed in an aircraft?
Low internal resistance intensified by high cell temperatures and a high current discharge/charge rate in a constant potential (voltage) charging system.
At what temperature is a battery charging adjustment necessary?
95 degrees F.
When a charging current is applied to a nickel cadmium battery, the cells emit gas
toward the end of the charging cycle