acid-base regulation
1/42
Earn XP
Description and Tags
block 2 week 1 ctb
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
sources of H+ in the body
volatile acids
14000 mmol H+ generated each day from aerobic metabolism and C02 production by tissues (H2CO3)
can leave solution and enter atmosphere
excreted by lungs
non-volatile (fixed/non-respiratory) ACIDS
70-100 mmol H+ generated each day from other metabolic processes forming (e.g sulphuric acid)
organic acids such as lactic acid/keto acids may also be formed in certain circumstances
excreted by kidneys
H+ regulation
acid-base regulation is the control of H+ concentration
as for other ions, a balance of intake, production and excretion is needed to maintain homeostasis (kidneys have a key role)
regulation of H+ is more complex and tighter than for other ions due to the effect of H+ on protein function
H+ is small and charged
alters protein activity, especially enzymes body wide effects as many physiological processes sensitive to small changes in H+
alters binding of other ions: e.g a low H+ increases Ca2+ binding to albumin
3 main mechanisms to minimise changes in pH:
buffer systems
lungs
kidneys
buffer systems
rapid chemical reactions that minimise any sudden changes in pH
unable to change overall body H+
lungs
can rapidly adjust excretion of C02
kidneys
can slowly adjust the excretion of H+ in the urine (altering body bicarbonate: HC03-) levels)
buffer systems: principles
a buffer is any substance that can reversibly bind H+
buffer + H+ ⇋ HBuffer
if H+ is added, buffer binds it to form HBuffer (removes H+)
if H+ removed, HBuffer releases H+ (adds H+)
rapidly adds or removes H+ so as to minimise overall changes in [H+] → as long as buffer is available
3 main buffer systems in the body
bicarbonate buffer system (extracellular)
H+ + HCO3- ↔ H2CO3 (carbonic acid)
phosphate buffer system (intracellular and in urine)
HPO42- + H+ ↔ H2PO4-
protein buffer system (mainly intracellular)
Pr- + H+ ↔ HPr
buffer systems: bicarbonate
Connects lung control of [CO2] to kidney control of bicarbonate [HCO3-] in acid-base balance – shows how the systems can compensate for each other
H+ + HCO3- ↔ H2CO3 ↔ H2O + CO2
![<ul><li><p><span><span>Connects </span><strong><span>lung</span></strong><span> control of [CO</span><sub><span>2</span></sub><span>] to </span><strong><span>kidney</span></strong><span> control of bicarbonate [HCO</span><sub><span>3</span></sub><sup><span>-</span></sup><span>] in acid-base balance – shows how the systems can compensate for each other</span></span></p></li><li><p><span><span>H</span><sup><span>+ </span></sup><span>+ HCO</span><sub><span>3</span></sub><sup><span>- </span></sup></span><span data-name="left_right_arrow" data-type="emoji">↔</span><span><span> H</span><sub><span>2</span></sub><span>CO</span><sub><span>3</span></sub><span> </span></span><span data-name="left_right_arrow" data-type="emoji">↔</span><span><span> H</span><sub><span>2</span></sub><span>O + CO</span><sub><span>2</span></sub></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/5a6968d8-c289-4d85-8e04-ff7ec9373462.png)
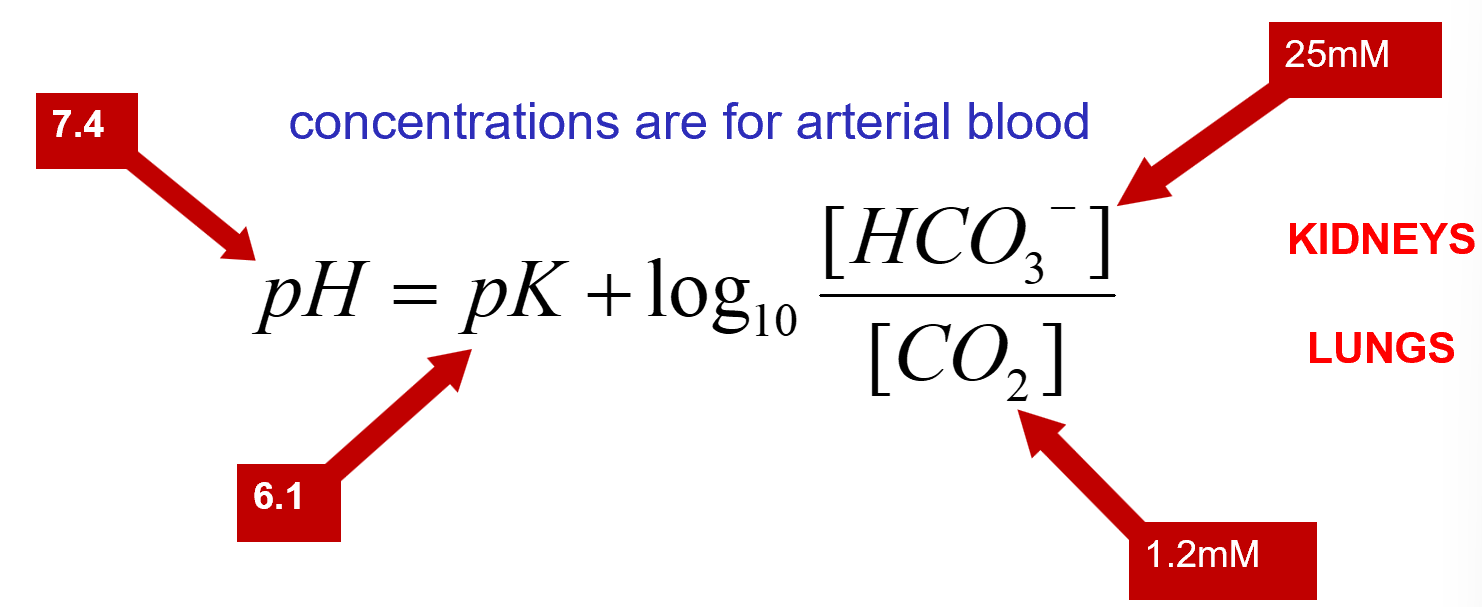
Henderson-Hasselbach Equation
this equation allows us to calculate pH based on measurements of [HCO3-] and [CO2]
pK is a constant for this reaction
[CO2] is calculated from partial pressure of CO2 (pCO2)
![<ul><li><p>this equation allows us to calculate pH based on measurements of [HCO<sub>3</sub><sup>-</sup>] and [CO<sub>2</sub>] </p></li><li><p>pK is a constant for this reaction</p></li><li><p>[CO<sub>2</sub>] is calculated from partial pressure of CO<sub>2</sub> (pCO<sub>2</sub>)</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/7e6c9bbb-fc74-4066-a53d-043a84cbab43.png)
concentration for arterial blood in Henderson-Hasselbach Equation
acid base regulation
maintaining pH depends on:
functional lungs to maintain CO2
functional kidneys to maintain HCO3
lungs
rapid response to alter CO2
kidney
slow response to alter HCO3 production and H+ excretion so as to restore pH
acid base balance
renal control of acid base
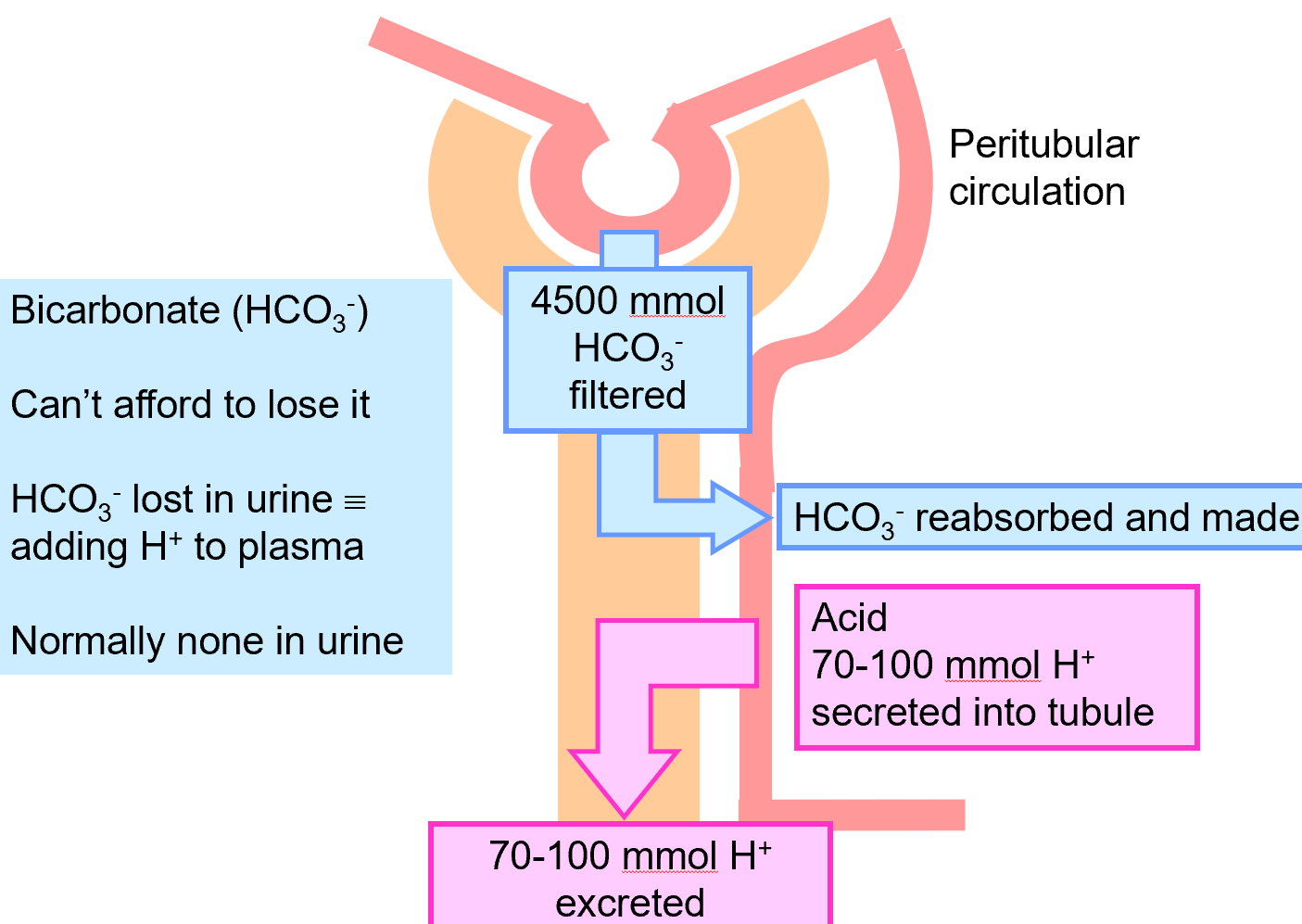
kidneys control extracellular fluid pH by adjusting the amount of H+ excreted in urine
to maintain acid-base balance, kidneys must excrete 70-100 mmol/day of H+ from non-volatile acid production
therefore: urine is usually acidic
kidneys must also reclaim the filtered HCO3- to avoid a reduction in HCO3-
the loss of H+ is equivalent to gain of HCO3-
renal control of acid-base
2 main processes by which kidneys regulate extracellualr fluid pH
reabsorption of filtered HCO3-
excretion of H+ (production of new HCO3-)
both processes rely on ability to secrete H+
peritubular circulation
reabsorption of filtered HCO3-
kidneys filter 4500mmol HCO3-
180 litres (filtrate/day) x 25 mmol/L [HCO3-]
usually must reabsorb all of this to maintain the blood HCO3- and avoid lowering the pH
majority reabsorbed in proximal convoluted tubule (85-90%)
reabsorption of filtered HCO3- in PCT
HCO3- can’t be directly transported from lumen: needs carbonic anhydrase and secreted H+
no net gain or loss of H+ or HCO3- so no change in acid-base status despite H+
secretion of H+ in late distal and collecting tubules
5% filtered HCO3- reabsorbed in late distal and collecting tubules by similar mechanism – method of H+ secretion into lumen differs
Uses H+ (and H+/K+) ATPase transporters in type A intercalating cells to pump H+ into tubular lumen
Activity can be stimulated by aldosterone and hypokalaemia
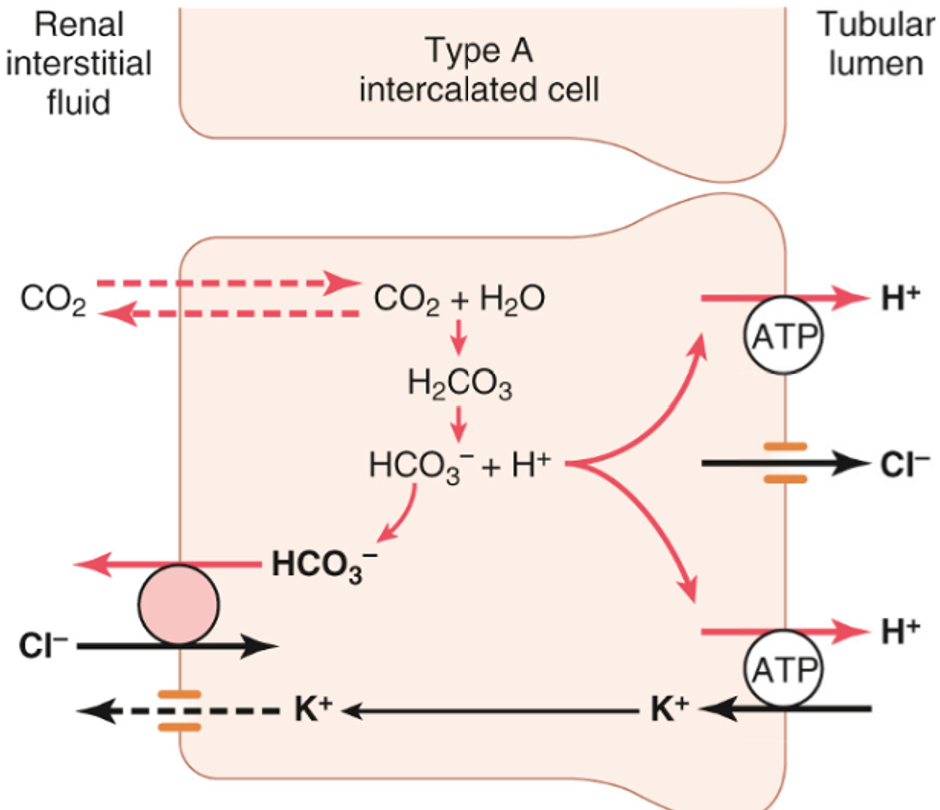
secretion of H+ in late distal or collecting tubules
H+ ATPase important in secreting H+ into tubule lumen – can generate an 800 fold H+ gradient, giving a minimum urinary pH of ~4.5
However this is still not sufficient alone to secrete all the 70-100mmol of non-volatile H+
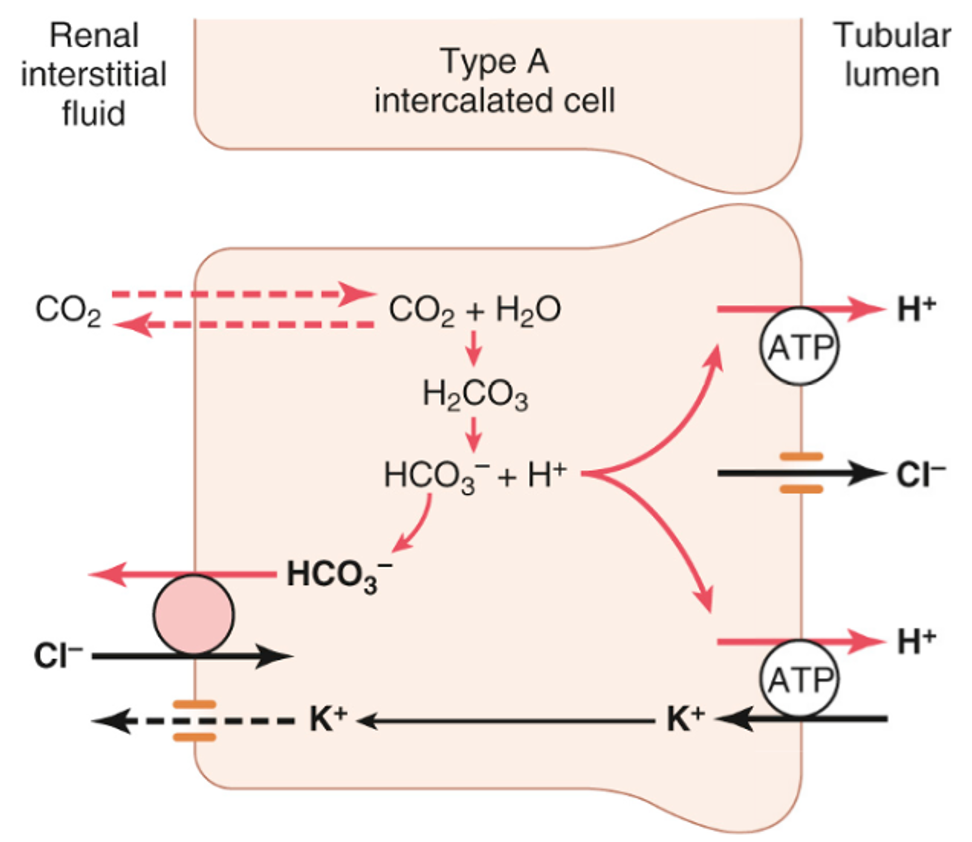
excretion of H+
Urinary buffers are essential both for comfort and to allow sufficient H+ to be excreted in the urine
The two main urinary buffers are phosphate and ammonia
The process of excreting H+ generates new HCO3-
Important to generate new HCO3- as some is consumed buffering the 70-100 mmol of non-volatile (fixed) acids produced each day
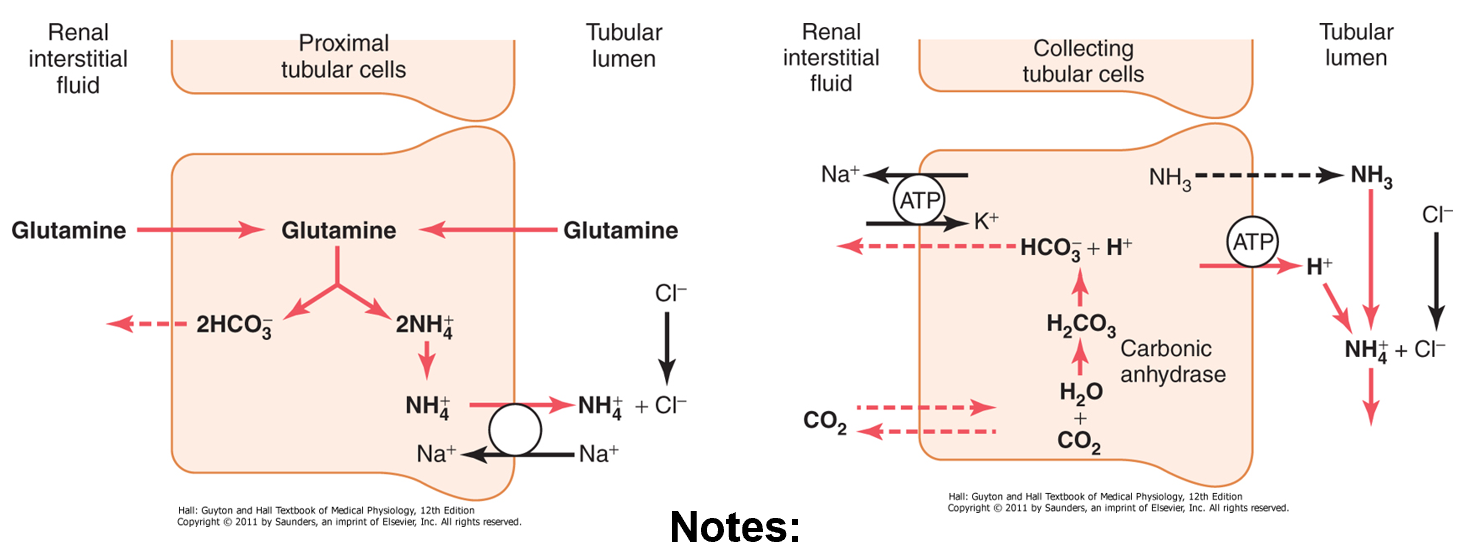
urinary phosphate buffer
filtered phosphate has 2 forms (monoprotic and diprotic) that create a buffer pair in renal tubular fluid
relative monoprotic form which is able to ‘pick up’ any excess secreted H+ in lumen and excrete it in urine
process of excreting H+ leads to production of HCO3- which passes into the blood
excretion of H+ by urinary phosphate buffer
Note that the H+ is excreted in combination with NaHPO4-
Note that as H+ is excreted in the urine, HCO3- passes into the interstitial fluid
urinary ammonia buffer
ammonium (NH4+) is synthesised from glutamine mainly in PCT cells (they contain glutaminase) as it is broken down to glutamate and then to alpha-ketoglutarate
ammonia and ammonium form a buffer pair
NH3+ + H+ ↔ NH4+
ammonia is eventually secreted mainly in collecting duct: ‘picks up’ excess secreted H+ and excretes it in urine as ammonium
process leads to production of HCO3-
excretion of H+ by urinary ammonia buffer
H+ is excreted in combination with NH3 as NH4+
as H+ is excreted in urine, HCO3- is being added to the blood
urinary ammonia buffer
can respond to body’s acid-base status
a decrease in pH stimulates renal glutamine metabolism leading eventually to increased H+ excretion (and vice versa)
renal responses are slower than lungs (requires protein synthesis/breakdown)
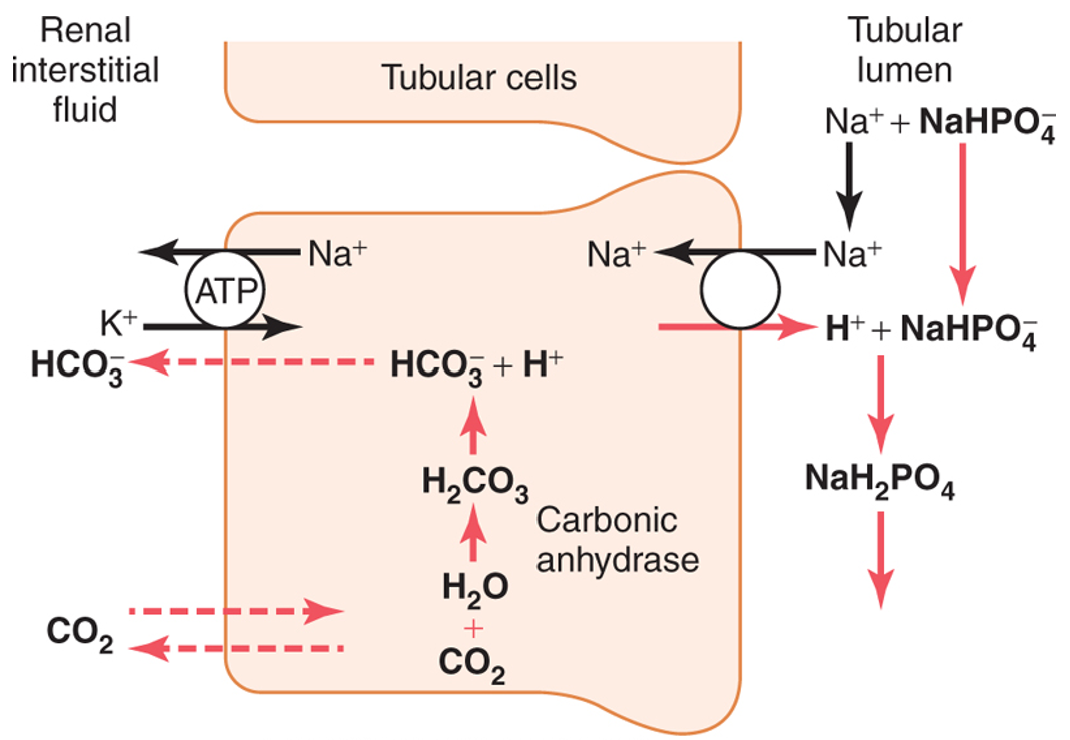
excretion of H+ by urinary phosphate buffer
H+ is excreted in combination with NaHPO4-
as H+ is excreted in the urine, HCO3- passes into the interstitial fluid
urinary ammonia buffer
ammonium (NH4+) is synthesised from glutamine mainly in PCT cells (they contain glutaminase) as it is broken down to glutamate and then α-ketoglutarate
ammonia and ammonium form a buffer pair NH3 + H+ ↔ NH4+
ammonia is eventually secreted mainly in collecting duct: ‘pics up’ excess secreted H+ and excreted it in urine as ammonium
process leads to the production of HCO3-
excretion of H+ by urinary ammonia buffer
H+ is excreted in combination with NH3 as NH4+
as H+ is excreted in urine, HCO3- is being added to the blood
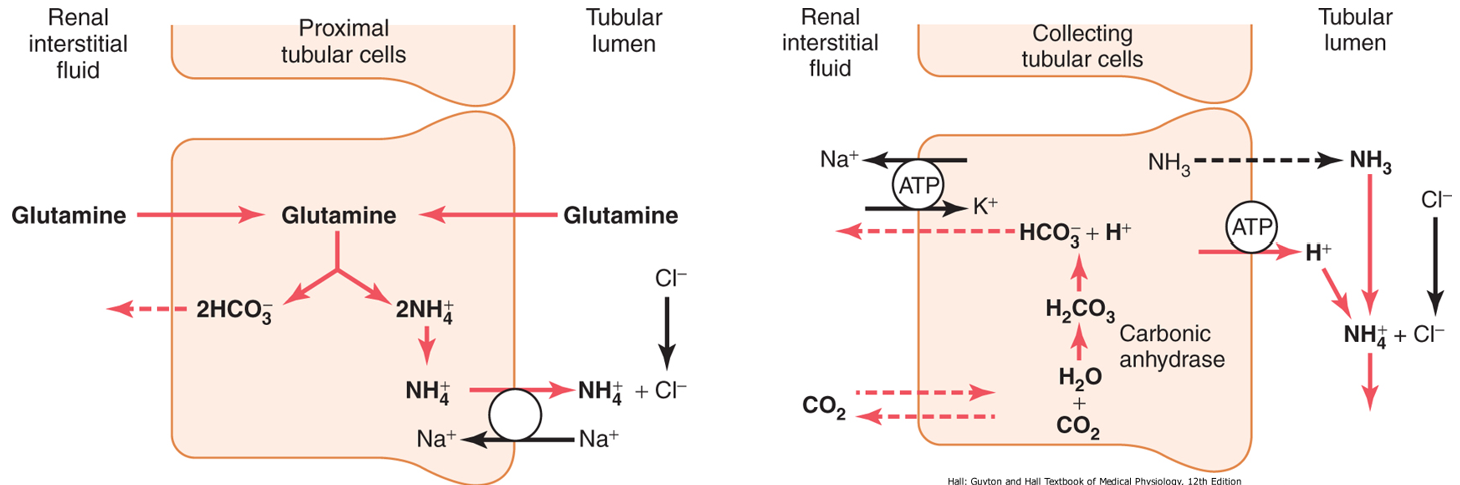
urinary ammonia buffer
the urinary buffer can respond to the body’s acid-base status
a decrease in pH stimulates renal glutamine metabolism leading eventually to increased H+ excretion
renal responses are slower than lungs: requires protein synthesis/breakdown
control of H+ secretion-
levels of H+ secretion control the amount of filtered HCO3- reabsorbed as well as new HCO3- produced
principally stimulated by:
an increase in pCO2 of the extracellular fluid
a decreased pH of the extracellular fluid
these mechanisms allow the kidneys to alter their H+ secretion (and in turn HCO3- reabsorption) appropriately
increased aldosterone levels and hypokalaemia can also stimulate H+ secretion
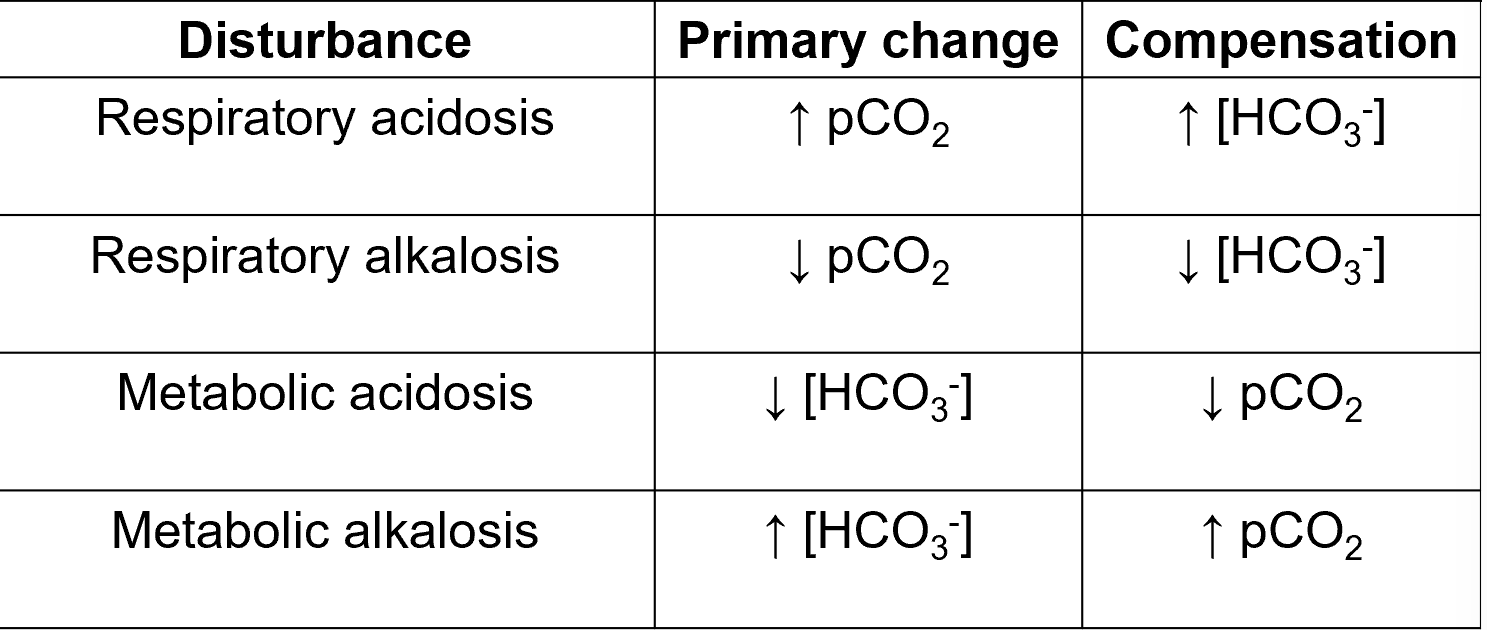
acid-base terminology
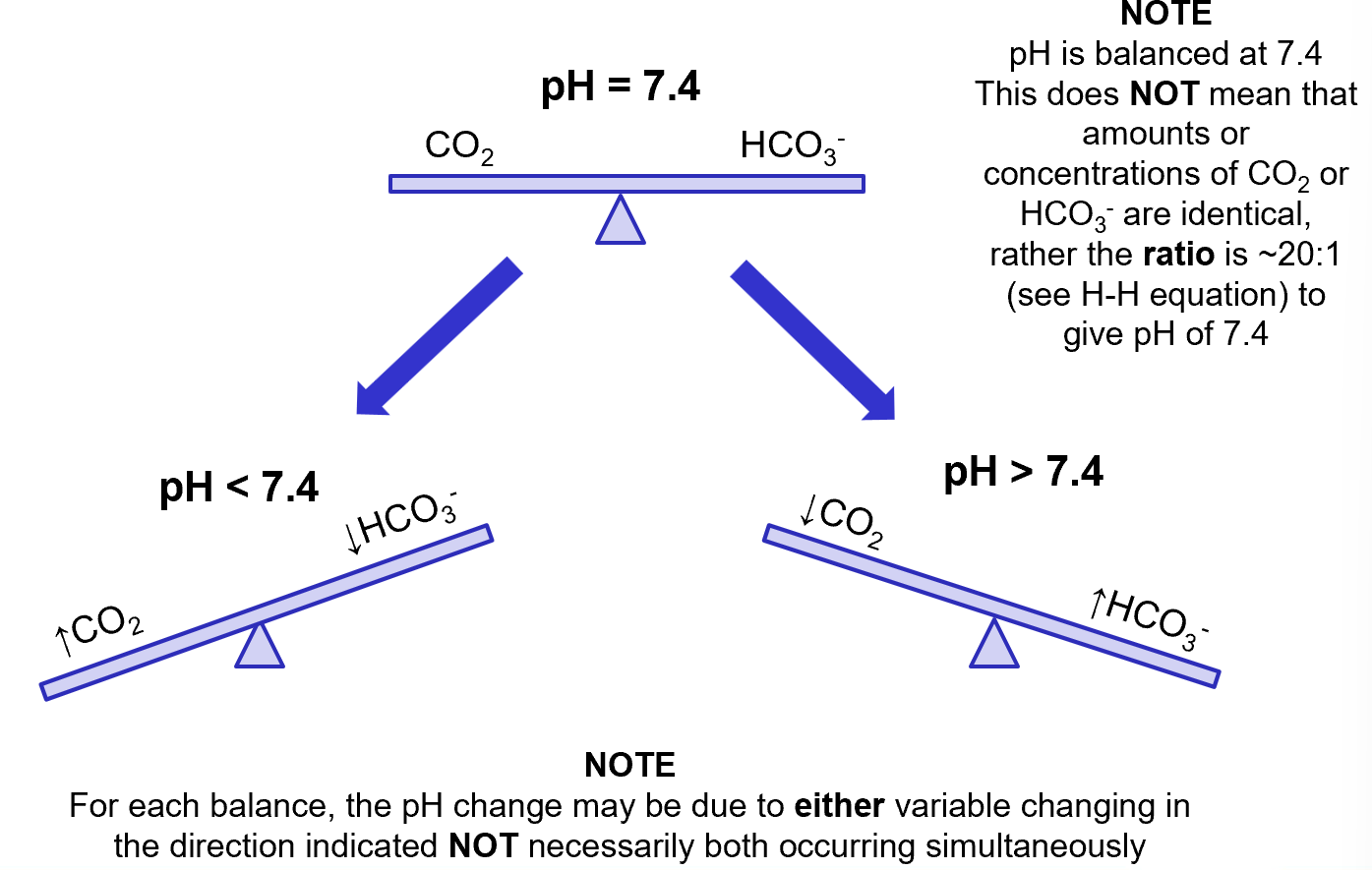
if a disease process alters the ratio of [HCO3-] to [CO2] then there will be a resulting change in pH
acidosis
any process that results in the blood becoming more acidic than normal
addition of acid and/or loss of alkali (base)
alkalosis
any process that results in the blood becoming more basic (alkaine) than normal
addition of alkali (base) and/or loss of acid
metabolic vs. respiratory problems
metabolic: the primary problem is affecting [HCO3-]
metabolic acidosis
metabolic alkalosis
respiratory: the primary problem is affecting CO2 excretion
respiratory acidosis
respiratory alkalosis
both acidosis and alkalosis signify underlying disease
compensation
because it is the ratio of [HCO3-] and [CO2] that gives us the pH, an abnormality affecting one parameter can be compensated to a certain degree by changes in the other
pH isn’t necessarily restored to normal but minimises the changes in pH: tries to restore back towards normal
in compensated disorders, both [HCO3-] and [CO2] values lie outside their normal ranges (in same direction- both raised and lowered)
respiratory acidosis
low pH due to increased CO2
causes: any disorder affecting the lungs, chest wall, nerves and muscles or CNS that leads to an inappropriate reduction in ventilation
compensation: slowly (days) by kidney to increase the production of bicarb
respiratory alkalosis
raised pH due to decreased CO2
causes: any disorder that leads to an inappropriate increase in ventilation: e.g anxiety and hyperventilation, high altitude
compensation: slowly by kidneys to decrease the production of bicarb
metabolic acidosis
low pH due to decreased [HCO3-]
causes: either addition of acid- exogenous (methanol) or endogenous (lactic acid or keto acids): failure of H+ excretion or loss of HCO3- (e.g severe prolonged diarrhoea)
anion gap can be used to narrow the differential diagnosis
compensation: rapidly by lungs to increase ventilation and therefore decrease CO2
metabolic alkalosis
raised pH due to increased [HCO3-]
causes: either addition of alkali or excess loss of H+ (e.g severe prolonged vomiting), excess aldosterone, e.g due to dehydration (stimulates H+ secretion in distal tubule)
compensation: rapidly by lungs to decrease ventilation and thus increase [CO2]
approach to treatment of metabolic acid-base
treat and correct the underlying problem whenever possible: most important
use substances to neutralise acid or base: controversial and senior decision
sodium bicarb to treat acidosis
ammonium chloride for alkalosis (uncommon)
interpreting acid-base
look at pH first
look at [HCO3-] and pCO2
if due to pCO2, it is a primary respiratory disorder
if due to [HCO3-] then it is a primary metabolic disorder
look for evidence of compensation
has the other value moved out of its normal range
acid base disorders summary