Unit 2 (Ch 2.4-2.8, 3, 4.1-4.4)
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
ionic
bond between oppositely charged ions, typically a metal + nonmetal, and involves a transfer of electrons
covalent
bond where electrons are shared, typically between 2 nonmetals
perspective drawing
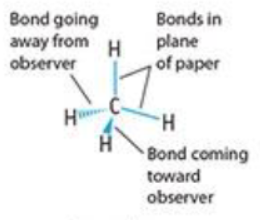
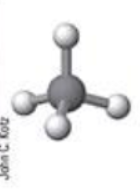
ball and stick model
space-filling model

+1
group 1 oxidation state
+2
group 2 oxidation state
+3
group 3 oxidation state
N/A
group 14 oxidation state
-3
group 15 oxidation state
-2
group 16 oxidation state
-1
group 17 (halogens) oxidation state
0
group 18 (noble gases) oxidation state
Zn and Cd oxidation state
+2
silver oxidation state
+1
Hg22+
Mercury (I)
NH4+
Ammonium
NO2-
Nitrite
NO3-
nitrate
SO32-
sulfite
SO42-
sulfate
HSO4-
hydrogen sulfate (aka. bisulfate)
OH-
hydroxide
CN-
cyanide
PO43-
phosphate
HPO42-
hydrogen phosphate
H2PO4-
dihydrogen phosphate
NCS- or SCN-
thiocyanate
CO32-
carbonate
HCO3-
hydrogen carbonate (aka. bicarbonate)
ClO-
hypochlorite
ClO2-
chlorite
ClO3-
chlorate
ClO4-
perchlorate
BrO4-
perbromate
IO4-
periodate
IO3-
iodate
BrO3-
bromate
IO2-
iodite
BrO2-
bromite
BrO-
hypobromite
IO-
hypoiodite
H3O+
hydronium
C2O42-
oxalate
C2H3O2-
acetate
MnO4-
permanganate
Cr2O72-
dichromate
CrO42-
chromate
acid
a substance with at least one H+ ion attached to anion, dissolved in water
prefix of “hydro”
acid ends in -ic
anion ends in -ate (contains oxygen)
acid ends in -ous
anion ends in -ite (contains oxygen)
alkanes
only contain single bonds between C and H
carboxylic acid
contains COOH group
alcohols
contain OH groups
amines
contain C-N bonds. occur when H atoms in ammonia (NH3) are replaced with organic groups
molar mass
the mass in grams of 1 mole of molecules
mass percent
the percentage by mass of a component of a mixture or an element of a compound
empirical formula
formula that contains the simplest ratio possible of atoms
molecular formula
the exact formula of a molecule; depends on molar mass
mono
1
di
2
tri
3
tetra
4
penta
5
hexa
6
hepta
7
octa
8
nona
9
deca
10