acids, bases and salts
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
61 Terms
acid → definition
an acid is a substance that can donate a proton (H^+) to another substance
base → definition
a base is a substance that can accept a proton (H^+) from another substance
examples of acids
hydrochloric acid (HCl) → H+ and Cl-
sulfuric acid (H_2SO_4) → H+ and SO_4^{2-}
nitric acid (HNO_3) → H+ and NO_3^-
phosphoric acid (H_3PO_4) → H+ and PO_4^{3-}
carbonic acid (H_2CO_3) → H+ and CO_3^{2-}
ethanoic acid (CH_3CO_2H) → H+ and CH_3CO_2^-
examples of alkalis
sodium hydroxide (NaOH) → OH- and Na+
potassium hydroxide (KOH) → OH- and K^+
calcium hydroxide (Ca(OH)_2) → OH- and Ca^{2+}
aqueous ammonia (NH_3) → OH- and NH_4+
aqueous ammonia as a base
NH_3 (aq) + H_2O (l) ⇌ NH_4+ (aq) + OH- (aq)
aqueous ammonia can dissolve in water and take in protons
strong acid → definition
a strong acid is one which dissociates fully in solution to give protons(H^+)
strong acid
degree of dissociation: approximately 100%
type of arrow used in dissociation equation: → (single arrow)
monobasic: HNO_3
dibasic: H_2SO_4
monobasic → definition
one mole of H^+ is dissociated per mole of an acid
dibasic → definition
2 moles of H^+ is dissociated per mole of an acid
strong base → definition
a strong base is one which dissociates fully in solution to give hydroxide ions (OH^-)
strong base
degree of dissociation: approximately 100%
type of arrow used in dissociation equation: → (single arrow)
monobasic: KOH
dibasic: Ca(OH)_2
weak acid → definition
a weak acid is one which dissociates partially in solution to give protons (H^+)
weak acid
degree of dissociation: <<< 100%
type of arrow used in dissociation equation: ⇌ (half arrow)
examples: organic acids
weak base → definition
a weak base is one which dissociates partially in solution to give hydroxide ions (OH^-)
weak base
degree of dissociation: <<< 100%
type of arrow used in dissociation equation: ⇌ (half arrow)
examples: ammonia
strength → definition
strength of an acid is a measure of the extent/percentage of dissociation of an acid in solution
concentration → definition
concentration of an acid is the number of moles of undissociated acid per unit volume
power of hydrogen (pH) → definition
the pH of a solution is defined as the negative logarithm to the base 10 of the concentration of hydrogen ions [H^+] in solution in mol dm^{-3}
formula for pH
pH = -log_{10}[H^+]
[H^+] = 10 ^{-pH}
pH value and concentration of hydrogen ions [H^+]
the larger the pH value, the smaller the [$H^+$]
acidic: more H^+ than OH^- → lower pH
alkali: more OH^- than H^+ → higher pH
pH scale
at 25°C:
neutral solution: [H^+] = [OH^-] → pH = 7
acidic solution: [H^+] > [OH^-] → pH < 7 (1-6)
alkaline solution: [H^+] < [OH^-] → pH > 7 (8-14)
indicators → definition
indicators are dyes or a mixture of dyes which change colour when added to acids or alkalis
physical properties of acids
have a sour taste
have pH less than 7
turn damp blue litmus paper red
may be solids (e.g. citric acid), liquids (e.g. concentrated sulfuric acid) or gases (e.g. hydrogen chloride gas)
can conduct electricity
can act as charge carriers as they exist as free mobile ions in aqueous solutions
physical properties of alkalis
have a bitter taste and feel soapy
have pH greater than 7
turn damp red litmus paper blue, turn universal indicator blue or violet
can conduct electricity
can act as charge carriers as they exist as free mobile ions in aqueous solution
neutralisation → definition
neutralisation refers to the reaction between an acid and a base to form salt and water
common operating conditions for Haber process
high pressure of 250 atm → force the reaction in a certain direction
moderate temperature of 450°C → reaction itself produces a lot of heat
iron catalyst
molar ratio of N_2 : H_2 is 1 : 3
acid-base nature of period 3 oxide
trend: the nature of the oxides change from basic (metal oxides) to amphoteric to acidic (non-metal oxides) across the periods
group 1 and group 2 → metal oxides
elements: Na (group 1) and Mg (group 2)
formula: Na_2O and MgO → giant ionic lattice (structure)
acid-base nature: basic
dissolves to form alkalis
behaves as bases which can react with acids
group 13 → metal oxide
elements: Al
formula: Al_2O_3 → giant ionic lattice (structure)
acid-base nature: amphoteric
properties of both acids and base → reacts with both acids and bases
group 14, group 15 and group 16 → non-metal oxide
elements: Si (group 14), P (group 15) and S (group 16)
formula: SiO_2; P_4O_6, P_4O_10; SO_2, SO_3 → covalent
P_4O_6: phosphorus (III) oxide
P_4O_{10}: phosphorus (V) oxide
acid-base nature: acidic
dissolves in water to form an acid
behaves as acids which can react with bases
basic oxides
can react with acids to form salt and water
usually insoluble in water (some can dissolve to form alkalis)
basic oxides and reaction with water
Na_2O + H_2O → 2NaOH
K_2O + H_2O → 2KOH
basic oxides and reaction with acids
Na_2O + 2HCl → 2NaCl + H_2O
K_2O + 2HCl → 2KCl + H_2O
MgO + 2HCl → MgCl_2 + H_2O
CaO + 2HCl → CaCl_2 + H_2O
acidic oxides
can react with bases to form salt and water
some can dissolve in water to form acidic solutions
SiO_2 not soluble in water due to its giant molecular structure and numerous strong covalent bonds
acidic oxides and reaction with water
SO_2 + H_2O → H_2SO_3 (sulfurous acid)
SO_3 + H_2O → H_2SO_4(sulfuric acid)
P_4O_6 + 6H_2O → 4H_3PO_3 (phosphorous acid)
P_4O_{10} + 6H_2O → 4H_3PO_4 (phosphoric acid)
acidic oxides and reaction with bases
P_4O_{10} (s) + 12NaOH (aq) → 4Na_3PO_4 (aq) + 6H_2O (l)
P_4O_6 + 8NaOH → 4Na_2HPO_3 + 2H_2O
SO_2 (g) + 2NaOH (aq)→ Na_2SO_3 (aq) + H_2O (l)
SO_3 (g) + 2NaOH (aq) → Na_2SO_4 (aq) + H_2O (l)
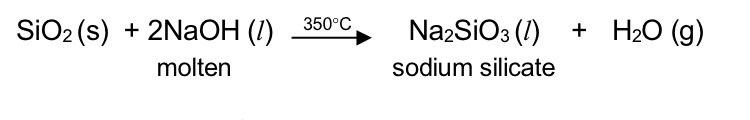
SiO_2 (s) + 2NaOH (l) - 350°C → Na_2SiO_3 (l) + H_2O (g)
amphoteric oxides
react with acids and bases to form salt and water
do not react with water
amphoteric examples
aluminium oxide (Al_2O_3)
lead (II) oxide (PbO)
zinc oxide (ZnO)
amphoteric oxides and reaction with acid
Al_2O_3 + 6HCl → 2AlCl_3 + 3H_2O
amphoteric oxides and reaction with base
Al_2O_3 + 2NaOH + 3H_2O → 2NaAl(OH)_4
neutral oxides
will not react with acids/bases → no reaction at all
examples: carbon monoxide (CO), water ($H_2O$), nitric oxide (NO) and nitrous oxide ($N_2O$)
reaction with acids and bases:
neutral oxides do not react with acids or bases
salts
ionic compounds
to determine type of reagents that could be used to form a salt → analyse the cations and anions of the salt
water of crystallisation in many hydrated salts can be driven off by heating the crystals of the salt strongly to form anhydrous salts
water of crystallisation
water bonded chemically within the crystals
hydrated salts
salts that contain water of crystallisation
anhydrous salts
salts that do not contain water of crystallisation
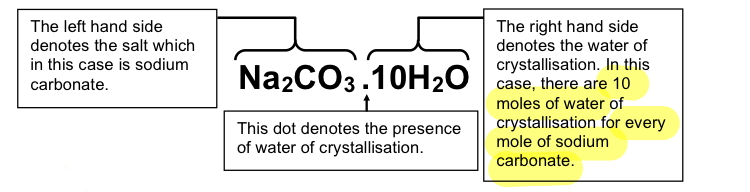
to name hydrated salts
the left hand side denotes the salt
the dot (.) denotes the presence of water of crystallisation
the right hand side denotes the water of crystallisation
formation of salts
acid + metal → salt + hydrogen gas
acid + carbonate → salt + water + carbon dioxide gas
acid + base → salt + water (neutralisation)
ammonium salt + base → salt + water + ammonia gas
need heat to dry off ammonia gas
2 soluble (aqueous) reagent → insoluble salt
reactivity series
K (most reactive) → Na → Ca → Mg → Al → Zn → Fe → Pb → H (H^+ acid) → Cu → Ag → Au (least reactive)
K and Na: highly reactive → explosive
K, Na, Ca, Mg, Al, Zn, Fe, Pb: more reactive than H → reacts with acids
Cu, Ag, Au: less reactive than H → will not react with acids
reaction of an acid and a metal
when a reactive metal (above hydrogen in reactivity series) reacts with a dilute acid, salt and hydrogen gas are formed
metal + acid → salt + hydrogen gas
extremely reactive metals in the reactivity series (group 1 metals: sodium and potassium) react violently and explosively
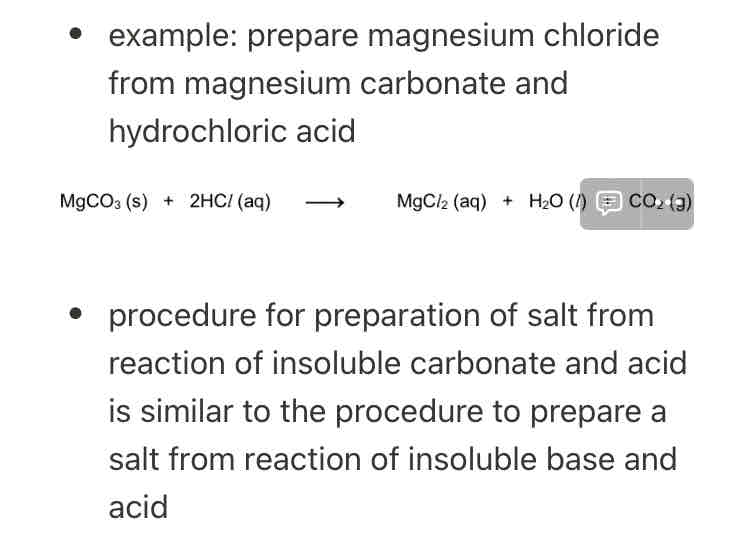
reaction of an acid and a carbonate/hydrogen carbonate
when a carbonate/hydrogen carbonate reacts with an acid, salt, water and carbon dioxide gas are formed
carbonate ({CO_3}^{2-}) + acid → salt + water + carbon dioxide gas
hydrogen carbonate ({HCO_3}^-) + acid → salt + water + carbon dioxide
reaction of an acid and a base
when an acid reacts with a base, salt and water are formed
acid + base → salt + water
when ammonia reacts with an a acid, only a salt is formed
acid + ammonia (NH_3) → salt (cation: {NH_4}^+)
reaction of an alkali and an ammonium salt
when an ammonium salt is warmed in the presence of an alkali, salt, water and ammonia gas are formed
ammonium salt ({NH_4}^+) + base - warm → salt + water + ammonia gas (NH_3)
precipitation reactions
insoluble salts can be formed by a precipitation reaction through the mixing of two soluble (aqueous) reagents
when an aqueous solution that contains the anion of an insoluble salt is mixed with an aqueous solution that contains the cation of that salt → the insoluble salt will precipitate out of the mixture
preparation of salt → guidelines
soluble salt: usually prepared from reactions with acids
insoluble salt: usually prepared by the precipitation method (mixing of two soluble salts)
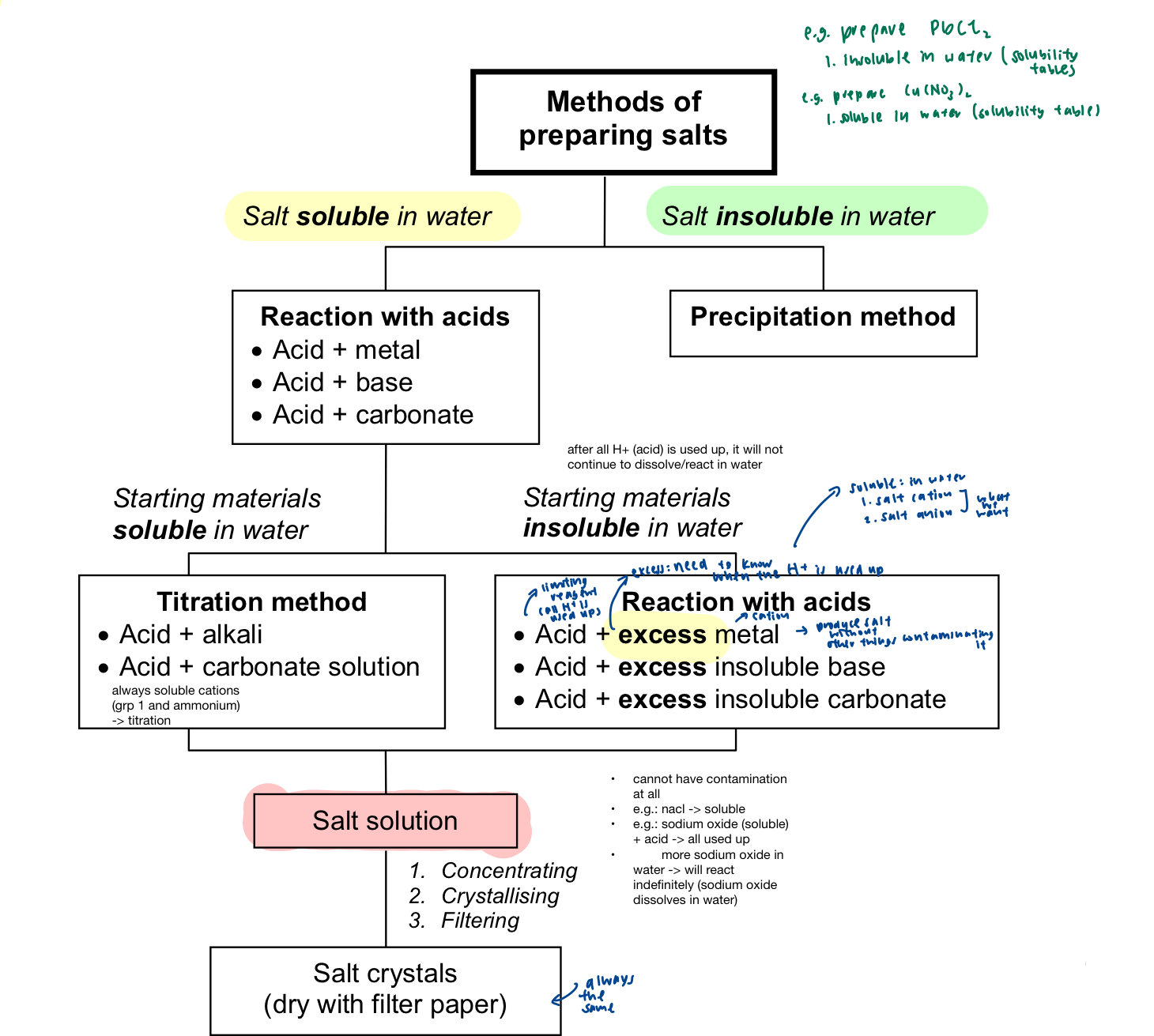
reaction with acids
if salt to be prepared is soluble in water while reactants are soluble:
reaction of acid with excess metal
reaction of acid with excess base (insoluble in water)
reaction of acid with excess carbonate (insoluble in water)
reaction of an acid and excess metal
to produce a salt that is soluble in water, excess metal is used such that the acid is completely used up → limiting reagent
hydrogen gas will escape to the surroundings → only the soluble salt and the excess metal will remain in the reaction container at the end of the reaction
the soluble salt and excess metal can be easily separated through filtration
this method is not suitable for:
very reactive metals such as sodium and potassium → react violently with acids
metals below hydrogen in the reactivity series such as copper, silver and gold → do not react with dilute acids

reaction of an acid and excess insoluble base
a salt can be prepared by a reaction between a base (metal oxide or metal hydroxide) and an acid
the procedure for preparation of salt from recall of insoluble base and acid is similar to the procedure to prepare salt from acid and excess metal

reaction of an acid with excess insoluble carbonate
carbonates and hydrogen carbonates can be soluble/insoluble in water
insoluble carbonate or hydrogen carbonate can be added in excess to an acid to produce a salt
reaction of an acid with a soluble base or carbonate
to overcome problem, an experiment has to be carried out to determine the exact volume of alkali (soluble base) that reacts with a know amount of acid, in the presence of an indicator → titration
the reaction is complete when the indicator changes colour. the volumes of acids and alkalis used are noted
to prepare the salt, the experiment is repeated with the same amount of acid and alkali used, in absence of indicator → can cause contamination to the salt prepared

preparation of insoluble salts via ionic precipitation reaction
criteria
choose 2 suitable ionic compounds that when combined will result in a precipitate of the desired solution
necessary to dissolve any solid starting material to get an aqueous solution first

sample question: why PbO(s) cannot be added directly to HI (aq) to prepare PbI_2 (s)
PbO + 2HI → PbI_2 + H_2O
reaction of PbO with HI forms insoluble PbI_2, which coats the surface of PbO solid. this protective layer prevents PbO from reacting with HI, leading to low yield of PbI_2