History and Fundamentals of Atomic Configurations and Balancing Equations in Chemistry
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
What do the coefficients in a chemical equation represent?
The number of particles or moles of reactants and products.
What is the balanced equation for the decomposition of water?
2H2O(l) → 2H2(g) + O2(g)
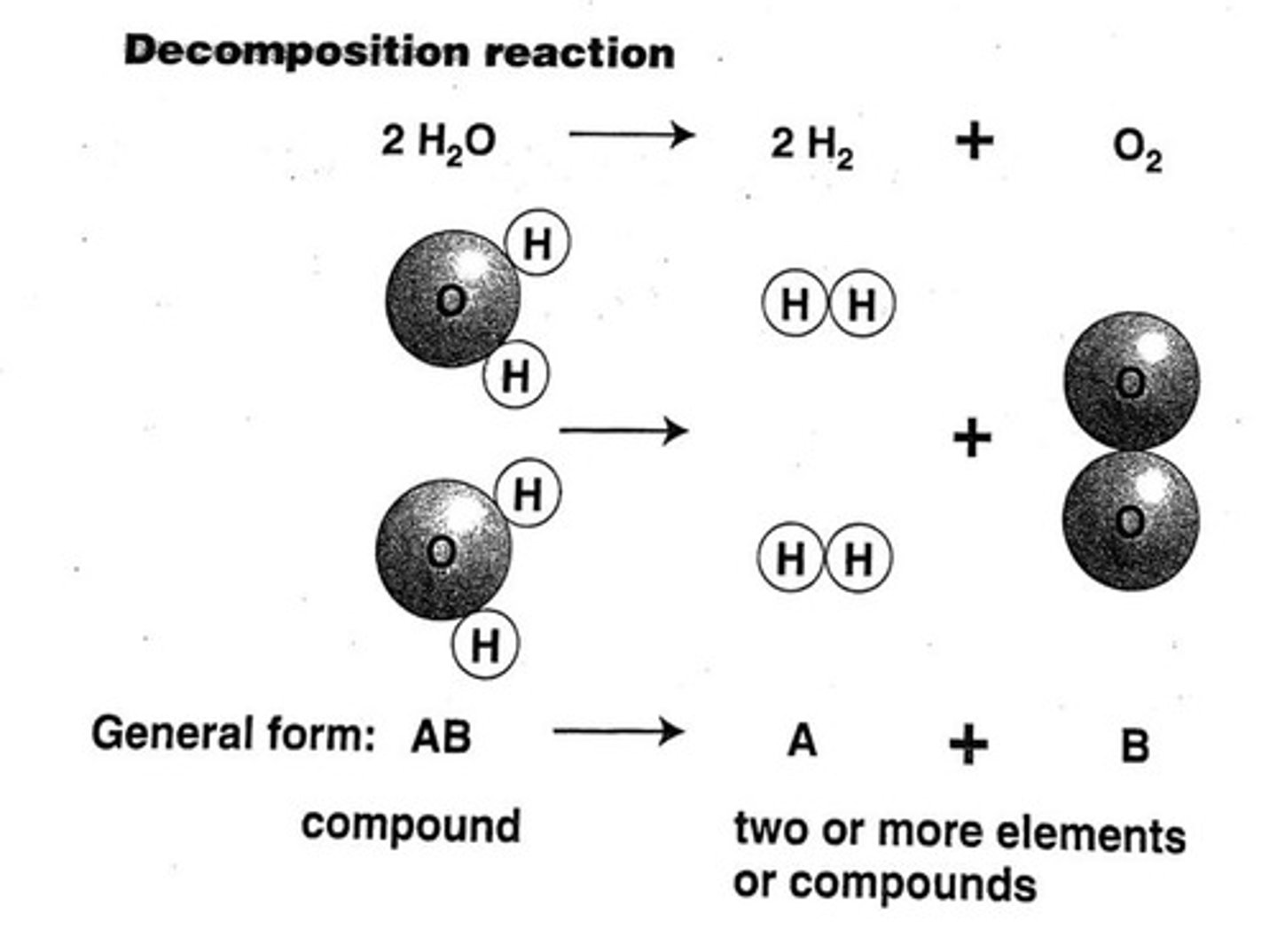
In the equation 2H2O(l) → 2H2(g) + O2(g), how many molecules of water are involved?
2 molecules of water.
In the equation 2H2O(l) → 2H2(g) + O2(g), how many moles of hydrogen gas are produced?
2 moles of hydrogen gas.
In the equation 2H2O(l) → 2H2(g) + O2(g), how many moles of oxygen gas are produced?
1 mole of oxygen gas.
What is stoichiometry?
The calculation of reactants and products in chemical reactions.
What is the significance of empirical and molecular formulas in chemistry?
They provide information about the composition and structure of compounds.
What is the process for converting mass to moles?
Mass to moles to empirical formula to molecular formula.
What is the relationship between percent composition and moles?
Percent composition can be used to determine mass, which can then be converted to moles.
What is the first step in determining the empirical formula from mass?
Convert mass to moles.
What does 'N/A is OK' refer to in the context of classifying equations?
It indicates that not all equations may fit into standard classifications.
What is combustion in chemistry?
A chemical reaction that typically involves the burning of a substance in oxygen.
What does the term 'beyond the mole road' imply?
It suggests exploring concepts in chemistry that extend past basic mole calculations.
What is the importance of balancing chemical equations?
It ensures the conservation of mass and that the same number of atoms are present on both sides of the equation.
What is the role of coefficients in a balanced chemical equation?
They indicate the relative amounts of reactants and products involved in the reaction.
How do you derive a molecular formula from an empirical formula?
By determining the molar mass and comparing it to the empirical formula mass.
What does the notation (l) indicate in a chemical equation?
It indicates that the substance is in the liquid state.
What does the notation (g) indicate in a chemical equation?
It indicates that the substance is in the gaseous state.