Bio Lecture Quiz #2
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
The seven groups of classification based on Linnaeus’ theory
(1) Kingdom, (2) Phylum, (3) Class, (4) Order, (5) Family, (6) Genus, (7) Species (Keep Pots Clean Or Family Gets Sick)
Organization of all living things (12)
(1) Atoms, (2) Molecules, (3) Organelle, (4) Cell, (5) Tissues, (6) Organ, (7) Organ system, (8) Organism, (9) Population, (10) Community, (11) Ecosystem, (12) Biome
Four characteristics of elements
(1) Most basic form of a substance, (2) Can’t be broken down into anything simpler, (3) Listed on the periodic table by atomic number, (4) Represented by 1-2 letters (e.g. Na)
How many naturally occurring elements are there vs. those made via nuclear reactions?
94 vs. 24
Atoms make up…
elements (they are the building blocks of everything)
Three parts of atoms
(1) Protons, (2) Neutrons, (3) Electrons
Three characteristics of protons
(1) Positive charge, (2) Located in nucleus, (3) Provides atomic number
Elements are defined by the number of…
protons
Three characteristics of neutrons
(1) No charge, (2) Located in nucleus, (3) Contributes to atomic mass w/ proton count
Isotopes
Atoms of the same element with different number of neutrons (does NOT change an element, just changes the atomic mass)
Neutrons block the repelling forces between…
protons, giving atoms stability
Radioactivity/radioisotopes
Results when neutrons can no longer counteract the repelling forces, and then atoms give it off (e.g. radioactive iodine used to diagnose thyroid disease affecting the metabolism; PET [Positron Emission Tomography] used to detect cancer)
Three characteristics of electrons
(1) Negative charge, (2) Found orbiting the nucleus, (3) Facilitates chemical bonds
In a neutral atom, the electron number is the same as…
proton number
What is the maximum number of electrons in each orbit of basic elements?
2 in first, 8 in second, 8 in third
For atoms to be stable, they must have a…
completed orbital (chemical bonding helps this)V
Valence electrons
Outer orbital electrons
Any element that ends in “ane” is…
flammable
Three types of chemical bonds
(1) Covalent, (2) Ionic, (3) Hydrogen
Covalent bonds do what?
Share valence electrons
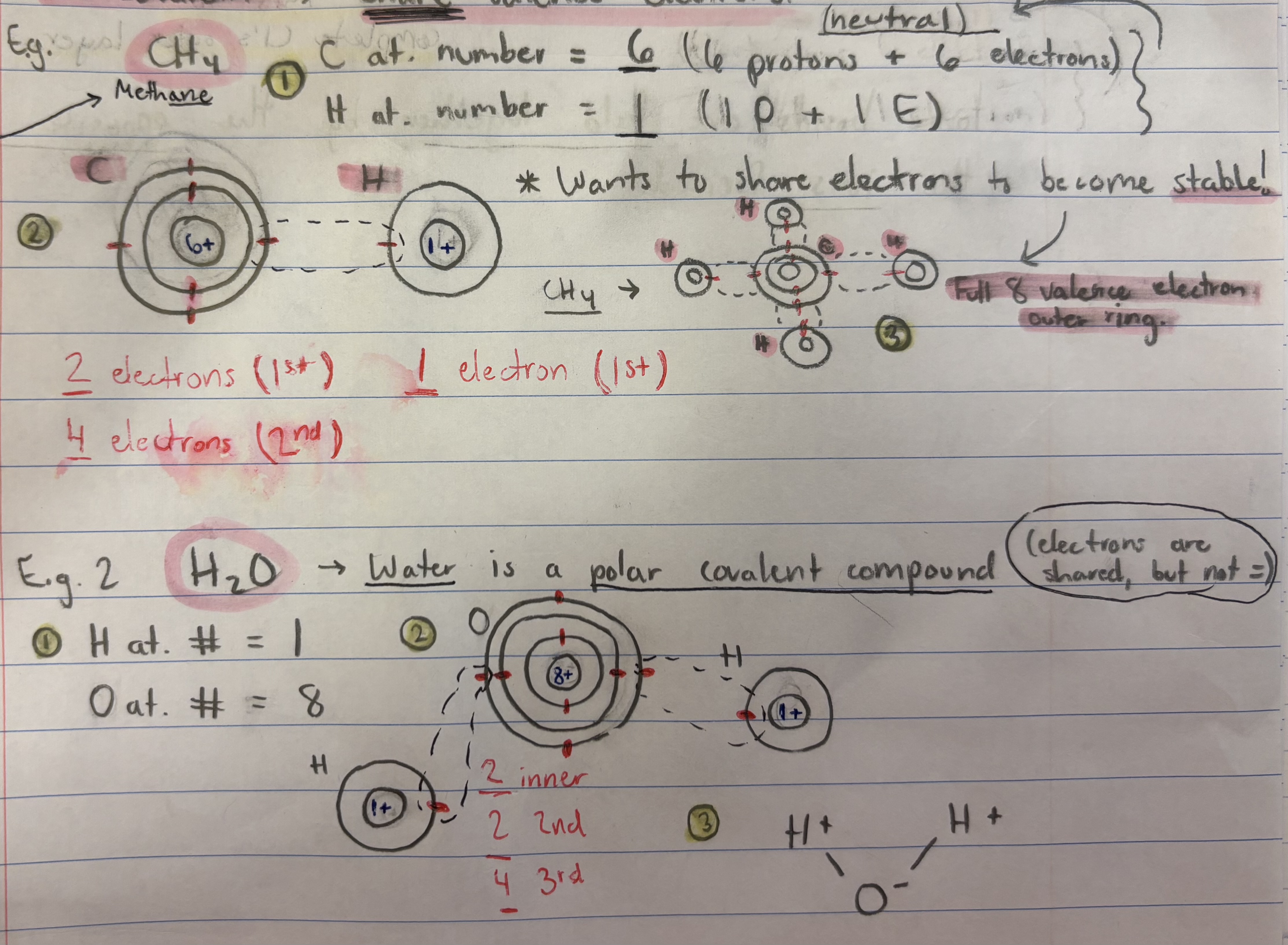
Process for determining electron count in covalently bonded elements:
(1) Figure out the atomic number for each part (e.g. H2O: H = 1, O = 8), (2) Draw out each part and count out how many electrons are in each section, (3) Determine where they will connect to fill valence electrons
Ionic bonds do what?
Exchange valence electrons
What is formed in ionic bonds?
Ions, which are charged atoms resulting from the loss/gain of electrons
Cations
Positive ions resulting from atoms losing electrons (formed from metal bonds)
Anions
Negative ions resulting from atoms gaining electrons (formed from nonmetal bonds)
Metal + Nonmetal =
Ionic bond
Nonmetal + Nonmetal =
Covalent bond
Ionic bonds are held together by the…
opposite charges forming on the ions
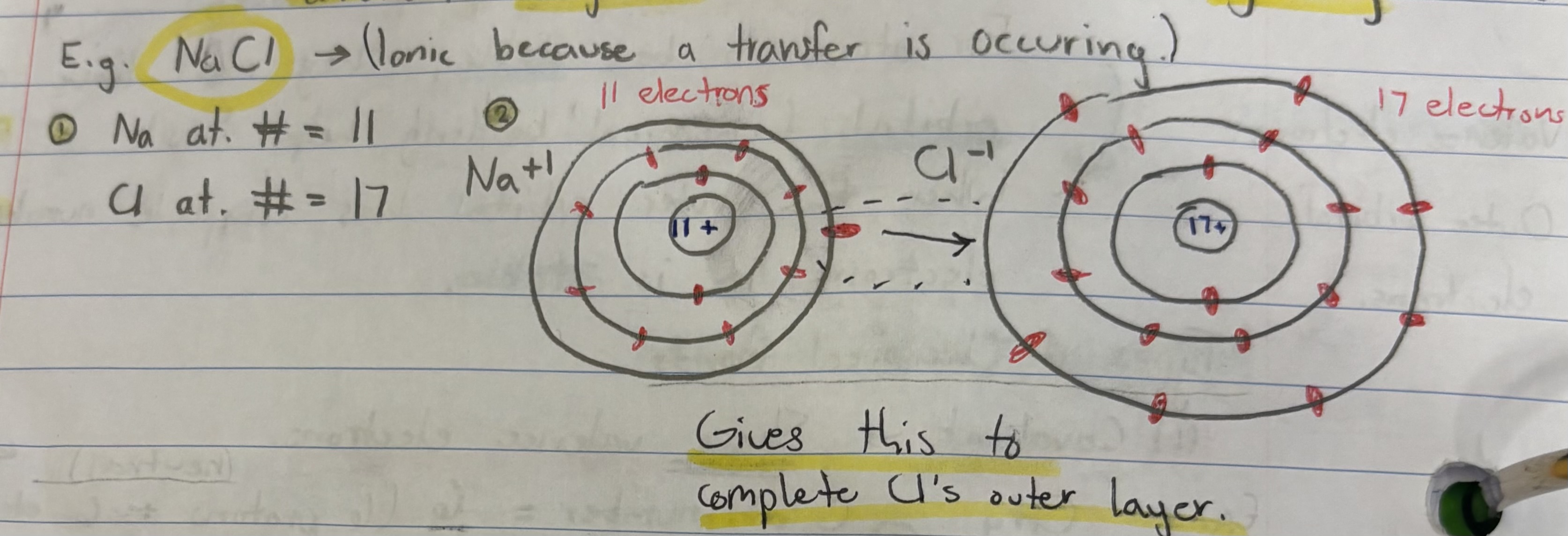
Process for determining electron count in ionically bonded elements:
(1) Figure out the atomic number for each part (e.g. NaCl: Na = 11, Cl = 17), (2) Draw out each part and count out how many electrons are in each section, (3) Determine where they will transfer to fill in missing valence electrons
What type of bonds do water molecules exhibit?
Polar covalent bonds
Polar covalent bonds
Oxygen develops a partial negative charge and the hydrogens develop a partial positive charge (b/c they don’t share their electrons evenly)
Hydrogen bonds
Forms between the partial positive charges on the hydrogen molecules and the negative charges of other molecules (e.g. H2O is a bent shape b/c the like charges of H are repelling each other)
The properties of water come from…
its hydrogen bonds
Four properties of water
(1) Water is cohesive (sticks to itself) & adhesive (sticks to other things), (2) Water is a good solvent (dissolves things), (3) Water has a high heat capacity (takes a lot to heat up), (4) Water expands when it freezes
Why is water’s cohesive & adhesive property important?
Surface tension & capillarity (ability of a liquid to be drawn up a narrow tube against gravity)
How does water’s solvent property work?
The water (solvent) dissolves the solutes and creates a solution by pulling apart many other molecules because of its opposing charges
What is an example of water’s solvent property?
Positively charged Na+ ions are attracted to the negatively charged side of the H2O molecule, while negatively charged Cl– ions are attracted to the positively charged side (thus dissolving it)
Why is water’s high heat capacity important?
Water requires a great deal of heat to be absorbed to change temperature, which is key for homeostasis (the internal conditions of an organism, e.g. a human, remaining unchanged despite changing external conditions)
Why is water’s lower density (expansion) when frozen important?
Due to hydrogen bonds, molecules become less tightly packed when solid, which is especially important for aquatic life when ice forms an insulating layer on the top of water bodies and helps preserve the life below
Water serves several critical functions within the body. What are they?
(1) Helps regulate body temp, (2) Universal solvent, (3) Cushions/transports/lubricates/creates high surface tension that allows body structures to cling to each other, (4) Aids in digestion
The pH scale measures…
acidity or basicity, a measure of the number of hydrogen ions (H+) in a solution (more hydrogen ions = lower pH, and vice versa)
Concentration
Amount of a particular solute that is dissolved in a given volume of fluid
pH ranges (0-14)
1-6.9 = acid (orange juice, stomach acid), 7 = neutral (water), 7.1-14 = base (cleaner, soap)
What is the importance of pH in our bodies?
Most biological processes occur within a narrow range of pH (around 7), and since most cellular reactions consume or produce H+ ions, pH changes would be very detrimental to many cells/reactions
Buffer
Chemicals which can either quickly absorb excess H+ ions or release H+ ions to keep the pH constant in our bodies (e.g. lungs, kidneys)