Topic 1 - Atomic Structure
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
What is the relative mass and charge of protons, neutrons and electrons?
Protons: Mass = 1 Charge = +1
Neutrons: Mass = 1 Charge = 0
Electron: Mass = Very Small/0 Charge = -1
What is the Atomic number and the Mass number?
Atomic Number - How many protons
Mass Number - Protons and Neutrons
What are Isotopes?
Isotopes - Different forms of an element which have the same number of protons but different number of neutrons.
How do you calculate the relative atomic mass of an element?
Mass = formula in image

What is a compound?
A substance formed form two or more elements chemically bonded together.
What is a mixture?
A mixture is similar to a compound but with no chemical bond.
What is chromatography?
It is a separation technique using dyes in ink.
See image for more info:

What is filtration?
Used to separate an insoluble solid from a liquid. Done using filter paper and a beaker.
What is Evaporation?
Used to separate a soluble solid from a solution. It is done by heating the solvent until crystals form.
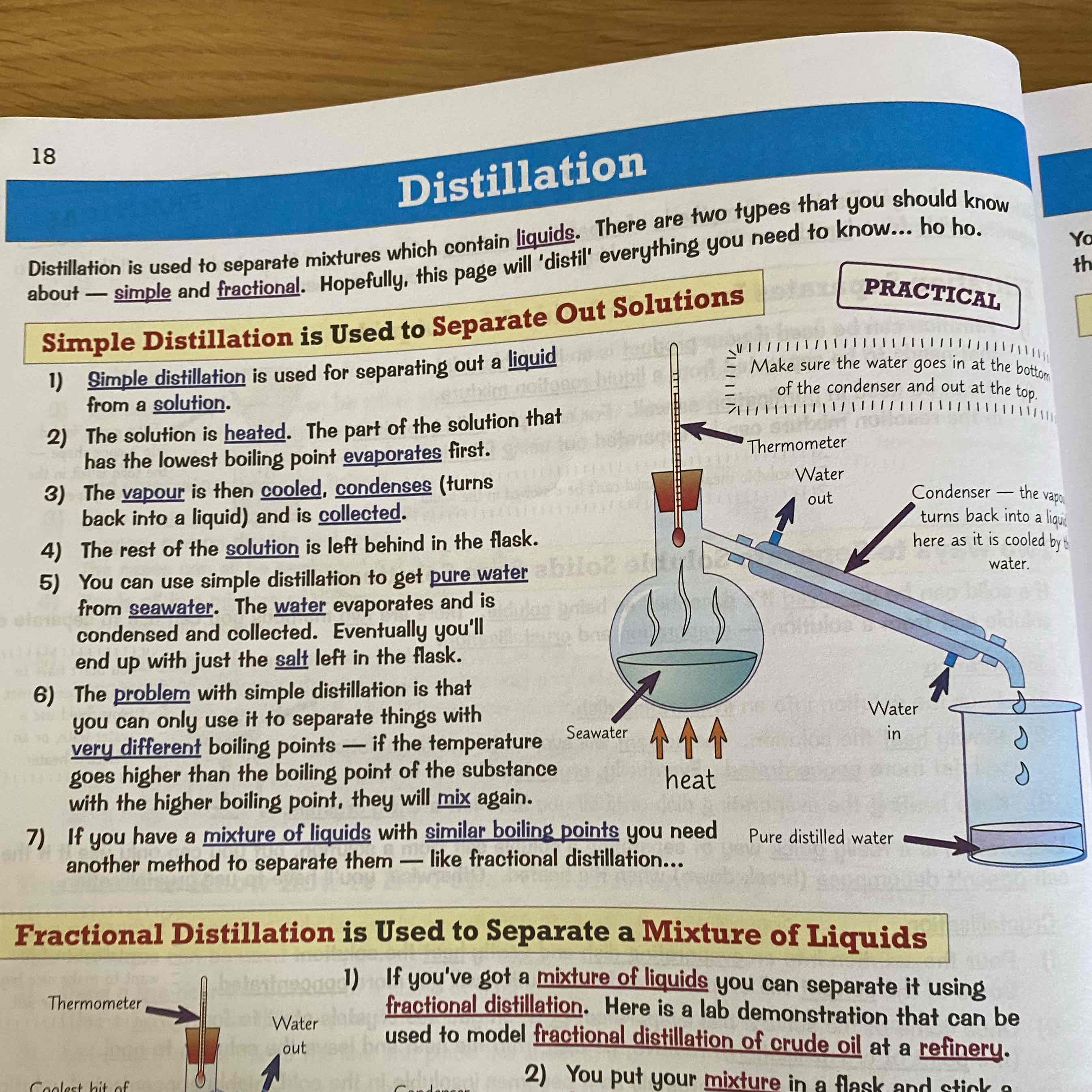
What is simple distillation?
Used to separate a liquid from a solution.
See image for more info:
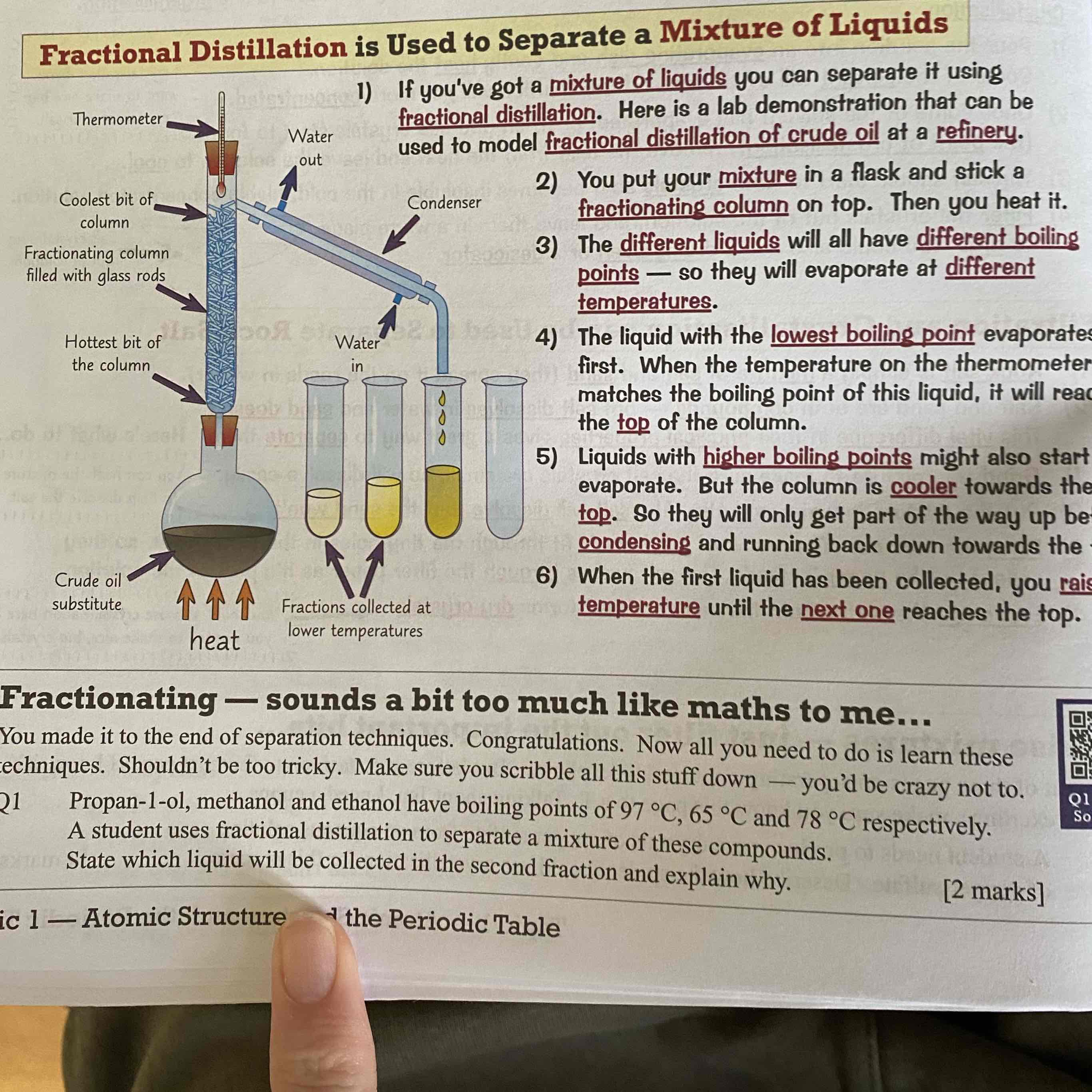
What is fractional distillation?
It is used to separate a mixture of liquids.
For more info see image:
The theory of the atom - describe:
John Dalton - 19th century- Solid spheres
J J Thompson - 1897 - Plum Pudding Model
Rutherford - 1909 - Gold Foil
Bohr - Nuclear Model - Electron Shells
James Chadwick - Neutrons
What are the electron shell rules?
Inner shells filled first
1st shell two electrons
Full shell can’t react - Noble Gas
What are some metal properties?
Strong
Conductors
High BP and MP
What are some non-metal properties?
Brittle
Lower density
Usually don’t conduct
Dull
What are the trends as you go down group 1?
Increasing Reactivity
Lower MP and BP
Higher mass
What are the trends as you go down group 7?
Less reactive
Higher MP and BP
Higher Mass
Why are group 0 called the noble gases?
As they are Colourless gases that don’t react as they have a full outer shell if electrons.
As you go down the group the is an increasing BP.