OCR A-Level Chemistry, Chapters 2-29 (without Transition Metals!)
1/884
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
885 Terms
adsorption in thin-layer chromatography is…
the process by which the solid silica holds the different substances in the mixture to its surface
formula for retention factor: Rf
distance moved by the compound / distance moved by solvent form
all forms of chromatography have a…
stationary phrase + a mobile phase
when is the stationary phase of gas chromatography?
high boiling liquid adsorbed into an inert solid support
when is the mobile phase of gas chromatography?
an inert carrier gas, like helium or neon.
how does gas chromatography work?
small amount of volatile mixture injected into apparatus
mobile carrier ga carries components in sample through capillary column, which contains the liquid stationary phase adsorbed onto the solid support
components slow down as they interact with the liquid stationary phase inside the column
the most soluble the component is in the liquid stationary phase, the slower it moves through the capillary column
components of the mixture are separated depending on their solubility in liquid stationary phase
compounds in mixture reach the detector at different times depending on their interactions with the stationary phase in the column
compound retained in the column time for the shortest time = lowest retention time + is detected first
what 2 things can be obtained from a gas chromatogram?
retention time → can identify components by comparing to known values
peak integrations (area under each peak) → determine concentration of components in sample
how to work out the concentrations of components from a gas chromatogram?
compared the peak integration with values obtained from standard solutions of the component →
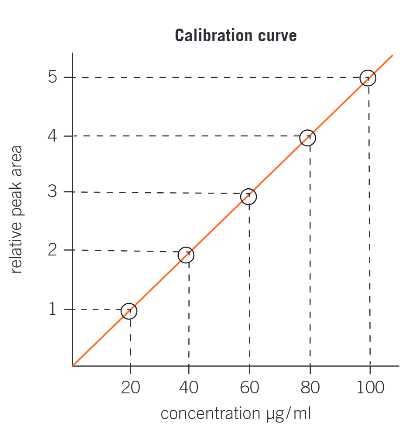
what is a calibration curve?
plotting relative peak area of a gas chromatogram against concentration, in μm/ml
qualitative test for an alkene?
add bromine water dropwise → bromine water is decolourised from orange to green
qualitative test for a haloalkane?
add silver nitrate + ethanol and warm to 50 degrees C in a water bath
chloroalkane = white precipitate
bromoalkane = cream precipitate
iodoalkane = yellow precipitate
qualitative test for a carbonyl:
add 2,4,-dinitrophenylhydrazine → orange precipitate
qualitative test for aldehyde:
add Tollens’ reagent and warm → forms silver mirror precip.
qualitative test for a carboxylic acid?
add aqueous sodium carbonate → effervescence
What is nuclear spin?
when there are an odd number of nucleons (protons and neutrons)
which are the two most relevant isotopes with nuclear spin?
1H and 13C
the 2 different spin states of nuclei have…
2 different energies.
what is the resonance of a nucleus?
when a nucleus absorbs energy, rapidly flipping between 2 spin states
what uses more energy, IR spec or NMR?
NMR → radio frequency has much less energy + the energy required is proportional to the magnetic field strength
what is chemical shift measured in?
parts per million
what is used as the standard reference against which all chemical shifts are measured?
Tetramethylsilane (TMS), (CH3)4Si
in an NMR spec, chemical shift value in ppm increases from…
right to left!!!!
what is chemical shift?
all atoms have electrons surrounding the nucleus → shifts the energy and radio frequency needed for nuclear magnetic resonance to take place
what can be worked out from a carbon-13 NMR spectrum?
the number of different carbon environments - from the number of peaks
the types of carbon environments present - from the chemical shift
carbons that are bonded to …. will have different chemical shifts:
different atoms or groups
if two carbon atoms are positioned … they will have the same chemical shift
symmetrically in a molecule → will contribute to the same peak
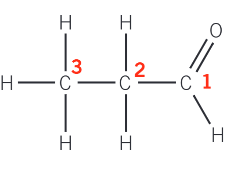
Carbon-13 NMR of propanal:
carbon-1 is part of the CHO functional group
carbon-2 is part of the CH2 group between a CH3 group and an aldehyde group
carbon-3 is part of a CH3 group, bonded to CH2CHO
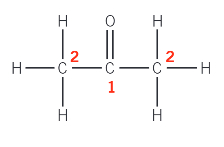
Carbon-13 NMR for propanone:
Carbon-1 is part of the C=O group.
The other two carbon atoms, both labelled 2, have the same environment. They are positioned symmetrically and can both be described in an identical way - each carbon atom is part of a CH3 group bonded to COCH3.
what 4 pieces of information can be told from proton NMR?
the number of different proton environments - from the number of peaks
the types of proton environments - from chemical shifts
the relative numbers of each type of proton - from integration traces or ratio numbers of the relative peak areas
the number of non-equivalent protons adjacent to a given proton - from the spin-spin splitting pattern.
what is the relative areas under each peak in proton NMR?
the ratio of the number of protons responsible for each peak
what is the integration trace of a proton NMR shown as?
an extra line or a printed number
how does a splitting pattern occur?
proton’s spin interacting with the spin of nearby protons that are in different environments.
the number of sub-peaks is…
one greater than the number of adjacent protons causing the splitting.
AKA → how many hydrogens are three bonds away?
spin-spin splitting occurs only…
if adjacent protons are in a different environment from the protons being split.
what is the relative intensity of signals on NMR spec?
simplest ratio between the number of different environments of Hydrogen
the 4 names of splitting patterns → how many Hydrogens are three bonds away from each of them?
singlet → 0 H’s 3 bonds away
doublet → 1 H 3 bonds away
triplet → 2 H’s 3 bonds away
quartet → 3 H’s bonds away
what is a multiplet?
a H-atom with 4 or more H’s that are 3 bonds away.
what kind of proton does split but is difficult to interpret? Forms one or more multiplets?
aromatic protons
if there is one splitting pattern…
there will be another!
-OH and -NH protons are not usually involved…
in spin-spin coupling → but they do still show up on an NMR spec! they are wide, pathetic peaks that are useless
what can be added to an NMR spec to detect -OH and -NH groups?
deuterium oxide, D2O → mixture is shaken, and a second spectrum is run.
what does deuterium oxide actually do?
replaces the -OH and -NH groups in a substance → this removes the pathetic peaks that they create on a proton NMR spectra.
equilibrium set up between methanol + D2O… (remember, D2O replaces the -OH and -NH molecules in a substance)
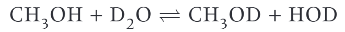
nitrile functional group:
-CN
haloalkane + NaCN/KCN in ethanol solvent →
nitrile
nucleophilic substitution of CN mechanism:
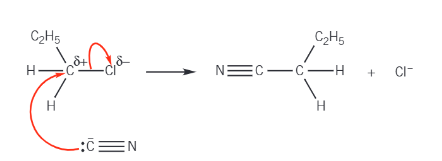
aldehyde/ketone + HCN →
hydroxynitrile
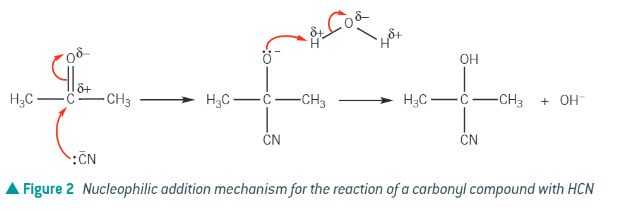
HCN is wayyyy too poisonous to use. Carbonyl + what → hydroxynitrile
carbonyl + NaCN/H2SO4 → Hydroxynitrile
Nucleophilic addition of CN to a ketone:
Nitrile + 2H2 → (Ni catalyst) →
amine
Nitrile + 2H2O + HCl → (heat) →
carboxylic acid + ammonium chloride
benzene + chloroalkane → (AlCl3) →
alkyl benzene + HCl
benzene + acyl chloride → (AlCl3) →
phenyl ketone + HCl
filtration under reduced pressure:
Connect one end of the pressure tubing to vacuum outlet or filter pump while attaching the other end of the rubber tubing to the Buchner flask
Fit Buchner funnel to the Buchner flask ensuring that there is a good + tight fit → usually obtained using Buchner ring / rubber bung
Switch on vacuum pump / tap to the which the filter pump is attached
Check for good suction by placing hand across top of funnel
Place piece of filter paper inside Buchner funnel + wet this with the same solvent used in preparing your solid. You should see the paper being sucked down against the holes in the funnel
To filter sample, slowly pour the reaction mixture from a beaker into the centre of the filter paper
Rinse out beaker with solvent so that all of the solid crystals collect in Buchner funnel
Rinse crystals in Buchner funnel with more solvent and leave them under suction for a few minutes so that the crystals start to dry.
Recrystallisation:
Pour a quantity of chosen solvent into a conical flask. If the solvent is flammable, warm the solvent over a water bath. If the solvent is water, place the conical flask on a tripod and gauze over a Bunsen + warm the solvent.
Tip impure sample into a second conical flask / beaker
slowly add solvent to the impure sample until it dissolves in the solvent. You should add the minimum volume of solvent needed to dissolve the solid.
Once the solid has dissolved, allow the solution to cool. Crystals of the desired product should form in the conical flask or beaker. When no more crystals form, filter the crystals under reduced pressure to obtain the dry crystalline solid.
A pure organic substance usually has a very … melting point
sharp
If a solid is impure…
It will melt over a wide range of temperatures + will have a higher melting point
Melting point determination:
Before taking the melting point of a solid you should ensure that the sample is completely dry and free flowing
Take a glass capillary tube or melting point tube. Hold one end of the capillary tube in the hot flame of a Bunsen burner. Rotate the tube in the flame until the end of the tube is sealed.
The capillary tube is allowed to cool, and is then filled with crystals to about 3mm depth. This is usually carried out by pushing the open end of the capillary into the solid sample to force some of the solid into the tube.
Once you have prepared your sample you will need to take its melting point. In schools + colleges, 1 of 2 methods are available: electrically heated melting point apparatus, or an oil bath.
Using an electrically heated melting point apparatus:
place the capillary tube containing the sample into a sample hole and a 0-300 degrees c thermometer hole of the melting point apparatus.
Using the rapid heating setting, start to heat up the sample whilst observing the sample through the magnifying window.
Once the solid is seen to melt, record the melting point. Allow the melting point apparatus to cool.
Prepare a second sample in a new capillary tube and place in the melting point apparatus and again heat up the sample.
As the melting point is approached, set to low and raise the temperature slowly whilst observing the sample. An accurate determination of the melting point can then be obtained.
Using an oil bath or Thiele tube method:
Set up the Thiele tube or oil bath
Attach the capillary tube containing the sample to a thermometer using a rubber band.
Insert the thermometer through a hole in the cork if using a Thiele tube or clamp the thermometer if using an oil bath. The end of the thermometer and the end of the capillary tube should dip into the oil.
Using a micro-burner, slowly heat the side arm of the Thiele tube or the oil bath whilst observing the solid. When the solid starts to melt, remove the heat and record the temperature at which all of the solid had melted. It is important to heat the oil slowly when approaching the melting point, and it is advisable to repeat the melting point determination a second time to ensure that you obtain an accurate value.
what is an aromatic amine?
the amine group, NH2, is attached directly to the aromatic ring.
when drawing the structure of an amine, you should show…
the lone pair
what makes an amine primary, secondary, or tertiary?
the number of alkyl or aryl (aromatic) groups attached to the nitrogen atom.
the suffix: ‘amine’ is only used when…
otherwise, the ‘ what’ prefix is used?
the amine group is on the end!
otherwise, the ‘amino’ prefix is used
When is ‘N-’ used in the naming of an amine?
When the NH group is attached to 2 different groups
When an amine acts as a base, what happens?
lone pair of electrons on the nitrogen can accept a proton
forms a dative covalent bond between the lone pair of electrons on the nitrogen atom and the proton
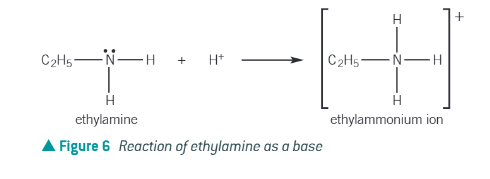
Amines are ….. → they neutralise acids to make salts.
bases
what does the salt created by an amine look like?
CH3CH2CH2NH3+Cl-
the neutralisation of acids by ammonia:
NH3 + HCl → NH4+Cl-
Producing a primary amine:
haloalkane + ammonia → ammonium salt (with halide ion)
CH3CH2CH2Cl + NH3 → CH3CH2CH2NH3+Cl-
ammonium salt (with halide ion) + NaOH → amine + sodium halide + water CH3CH2CH2NH3+Cl + NaOH → CH3CH2CH2NH2 + NaCl + H2O
what 2 conditions are required in the production of a primary amine? why?
Ethanol used as the hot solvent. This prevents any substitution of the haloalkane by water to produce alcohols.
Excess ammonia is used. This reduces further substitution of the amine group to form secondary and tertiary amines.
How to produce secondary amines:
haloalkane + primary amine → ammonium salt (with both alkyl groups wither side of the N)
ammonium salt + NaOH → secondary amine (with alkyl groups either side of the N) + H2O
how to produce tertiary amines?
further substitution of the secondary amine !
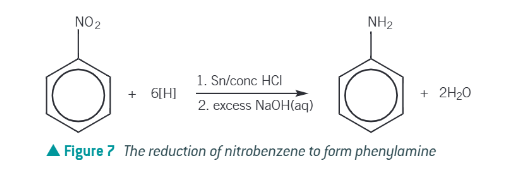
Preparation of aromatic amines:
Reduction of nitrobenzne:
heat under reflux with tin and hydrochloric acid
react the produced phenylammonium chloride with excess sodium hydroxide
what is an amino acid?
an organic compound containing both amine and carboxylic acid functional groups
what is an α-amino acid?
the amine group is attached to the second carbon atom → next to the carboxyl group
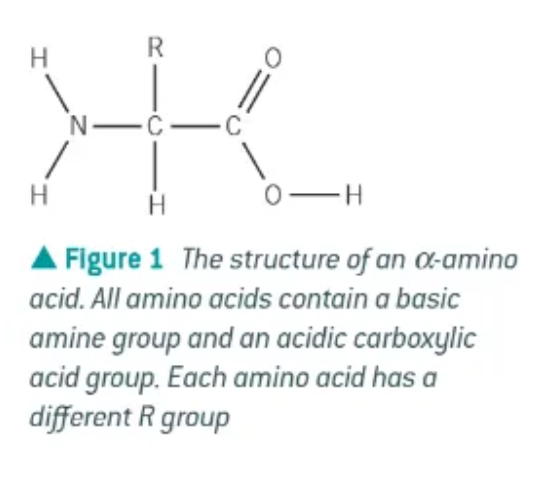
general formula of an α-amino acid:
RCH(NH2)COOH
what are β-amino acids and γ-amino acids?
β-amino acid = the amino group is attached to the 3rd carbon atom
γ-amino acid = the amino acid is attached to the 4th carbon atom
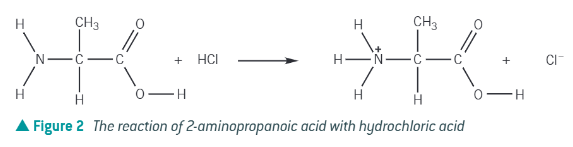
amino acid + acid →
ammonium salt + ion
acts as base, and accepts a proton!
amine end accepts a proton!
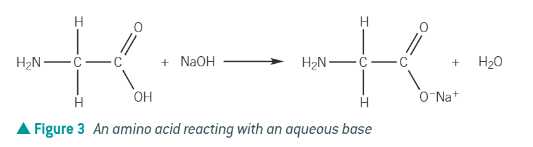
amino acid + alkali →
salt + water
carboxylic acid loses H+!
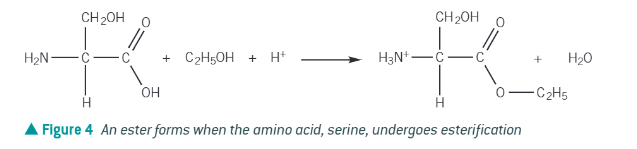
Esterification of amino acids with alcohols:
like carboxylic acids, react with alcohol + concentrated sulphuric acid to form an ester.
the amine group also accepts a proton too!!!!
what is a chiral carbon?
a carbon that is attached to 4 different atoms or groups of atoms.
Another name for optical isomers:
enantiomers
with the exception of H2NCH2COOH, all α-amino acids contain…
a chiral carbon centre → and therefore enantiomers!
in the structure, the chiral carbon centre is labelled with an…
asterisk
condensation polymerisation definition:
joining of monomers with the loss of a small molecule
usually water or HCl
requires 2 different functional groups
2 ways of making polyesters:
one monomer containing an -OH group and a -COOH group.
two monomers: one containing 2 -OH groups, and one containing 2 -COOH groups.
When drawing a section of polymer…
it’s important to leave open bonds at end each → it is normal to bracket it too!
some uses of polyesters:
electrical insulation
clothing
plastic PET bottles
Alternative way of making a polyester (using a diacyl chloride):
diacyl chloride + diol → polyester + water
2 ways of making polyamides:
one monomer: contains a COOH (or COCl) groups and one amine group
two monomers: one containing 2 COOH groups, and one containing 2 amine groups.
what happens when an amino acid undergoes condensation polymerisation with itself?
produces a polyamide
how to produce a polyamide with 2 different monomers:
diamine + dicarboxylic acid/dioyl chloride
Base hydrolysis of a polyester →
polyester → (NaOH / H2O) → +Na-COO xx COO-Na+ + diol
Acid hydrolysis of a polyester:
polyester → (H+ / H2O) → diol + dicarboxylic acid
(similar to the reverse of esterification!)
Base hydrolysis of polyamides:
polyamide → (NaOH / H2O) → diamine + +Na-COO(xx)COO-Na+ salt
acid hydrolysis of polyamides:
polyamide → (H+/H2O) → dicarboxylic acid + H3N+-(xx)-+NH3
what is a carbonyl functional group?
C=O
Aldehyde written in structural formula:
CHO
Ketone written in structural formula:
CO