History of the Atomic Model
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Democritus
Coined the term Atom and believed atom meant indivisible
Democritus
Did no experiments
Democritus
All elements have different shapes/sizes (no visual model)
Democritus
First to come up with the idea of atoms
Aristotle
Did not believe in atoms (ie, disagreed with Democritus)
Aristotle
Believed that all matter was continuous - idea lasted 2000 years
Dalton helped verify this
Law of conservation of mass - mass can’t be created or destroyed by ordinary chemical or physical reactions
Law of definite proportions
A compound contains the same proportions of mass regardless of size or source
Law of multiple proportions
If two or more compounds of the same two elements exists, then the ratio of moles are ratios of whole numbers
Dalton
All matter is made of atoms
Dalton
Atoms are an element are all the same, atoms of a different element will differ - was later proven wrong
Dalton
Atoms can’t be subdivided, created, or destroyed - was later proven wrong
Dalton
Atoms of different elements form compounds in whole number ratios
Dalton
In reactions, atoms are combined, seperated, or rearranged
Dalton’s theory
Isotopes and ions proved this theory wrong
Dalton’s theory
Nuclear reactions can split an atom proved this theory wrong
Dalton’s atomic model
Whose atomic model is this?
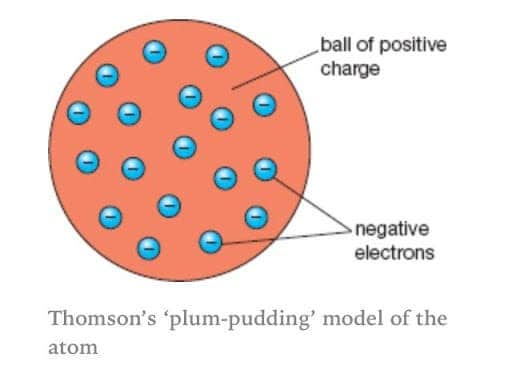
Thomson
Cathode ray experiment
Thomson
Measured the charge/mass ratio of cathode ray particles and found that this ratio is the same for all metals he tested as a cathode and any gas used.
Thomson
Concluded that cathode rays were made of identical negatively charged particles
Thomson
Discovered the electron
Thomson
“Plums” were negative because the cathode ray deflected towards the positive end
“Pudding” was mostly positively charged because the overall charge had to be neutral
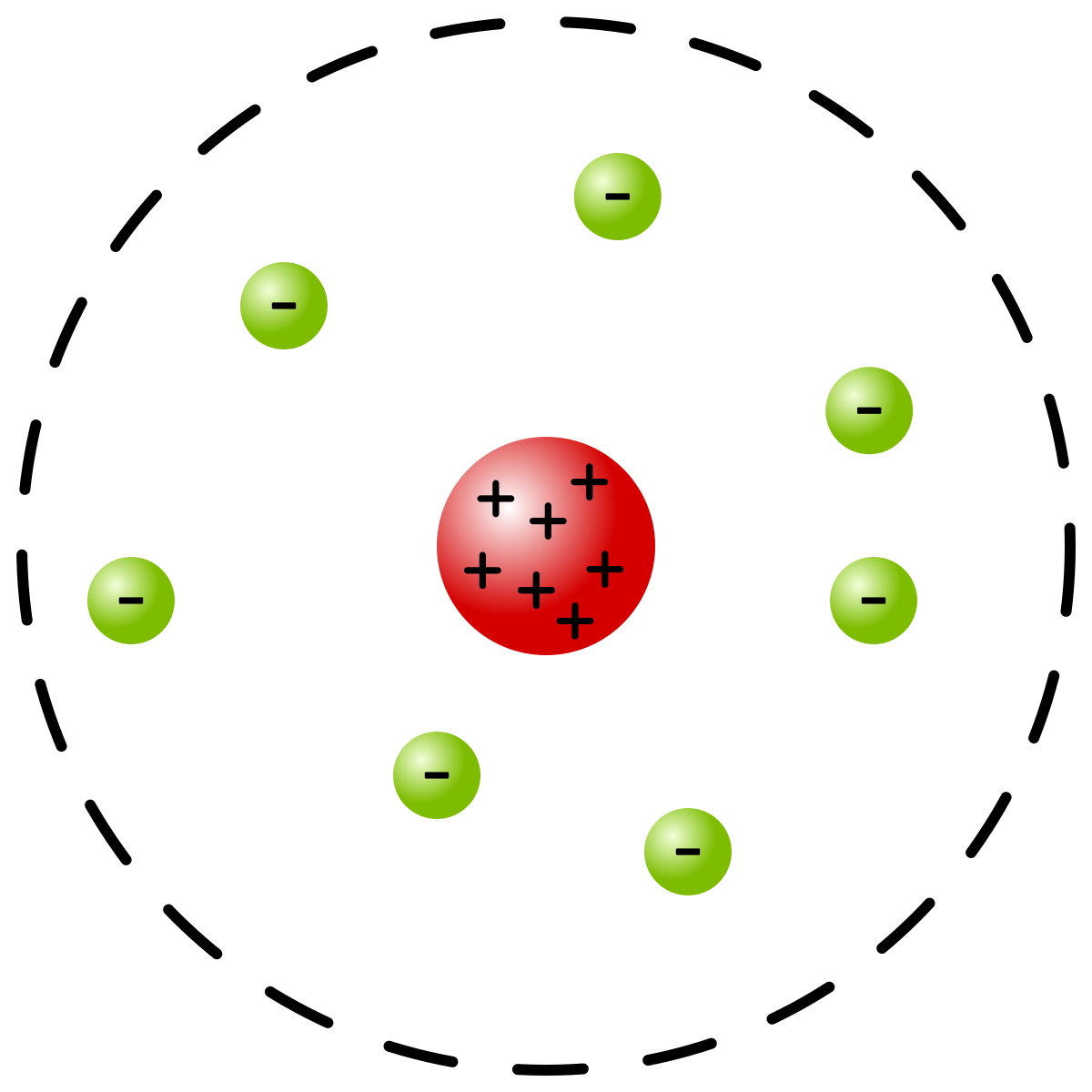
Rutherford
Shot alpha particles (positvely charged particles) through gold foil
Rutherford
Came to conclusion that there is a positive, densely packed center which repels the electrons
Rutherford
Discovered the nucleus
Rutherford
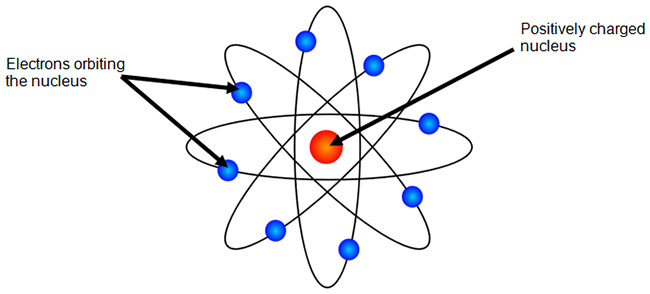
Which atomic model is this?
Bohr
Used Planck’s and Einstien’s work on energy
Borh
Determined that different electrons have different energy
Bohr
Showed the difference in energy using energy levels or orbitals
First to use energy orbits
Bohr
Lowest allowable energy state is the “ground state”
Bohr
When energy is added, electron moves to “excited state”
Bohr
When electron moves back to its normal energy level, it releases electromagnetic energy
Bohr
Which model is this?
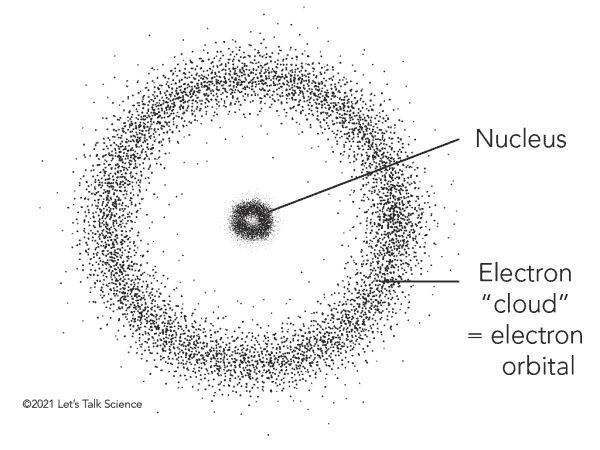
Electron Cloud model
Nucleus: contains protons and neutrons
Electrons: shown as a “cloud” indicating a “high probability of finding electrons”
Thomson
Determined the charge on electrons
Thomson
First model to show electrons