Ionisation energy
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Define first ionisation energy.
Energy required to completely remove one mole of electron from one mole of its gaseous atoms.
How does the Nuclear charge affect the ionisation energy?
Greater the nuclear charge the greater the attraction for the outer electrons from the nucleus
What is electron shielding?
Repulsion between electrons in different shells. Inner electron shells repel outer shell electrons
How does the electron shielding affect the ionisation energy?
Filled inner shells or sub-shells of electron as a sub-shield, outer electron shielded from nucleus attraction - decreases the ionisation energy.
How does the distance affect the ionisation energy?
Attraction decreases the further away the outer electron is from the nucleus - less attraction between nucleus and electrons
How is the ionisation energy down a group?
Decreases
Increased shells = increased shielding = further distance = less attraction = lower IE
How is the ionisation energy across a period
Increases
More protons = more attraction = high IE
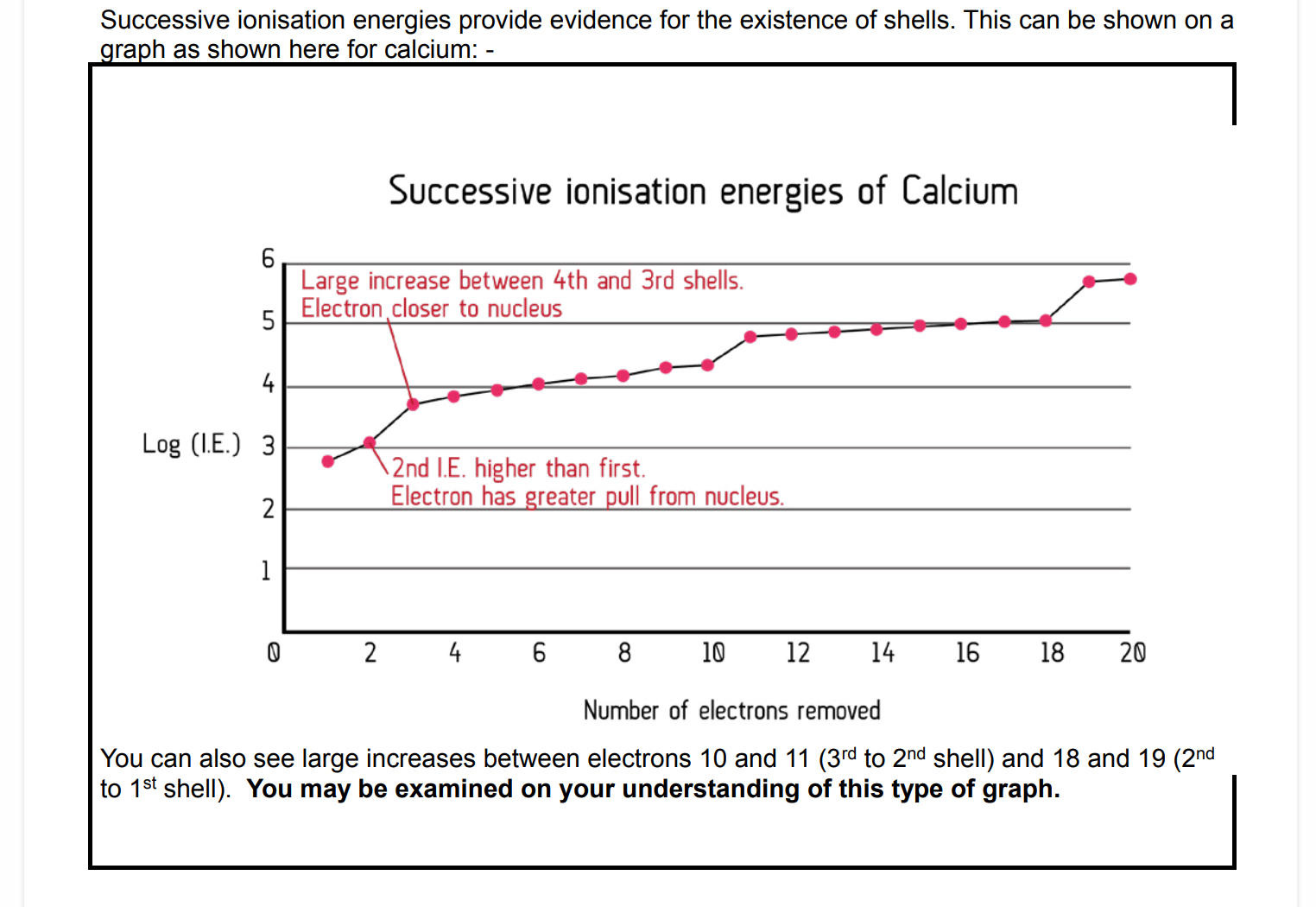
What is a successive ionisation energy?
Measure of the energy needed to remove each electron in turn until all the electrons are removed from am atom.
Why did successive ionisation energies always increase?
the number of protons stays the same, but they are effectively holding fewer and fewer electrons =
(greater effective nuclear charge)
each shell is drawn slightly closer into the nucleus as each electron is removed = (less electron to electron
repulsion)
as the distance between the nucleus and the electrons decreases, nuclear attraction increases.
Why is group 6 ionisation energy slightly less than group 5?
repulsion between the 2 electrons paired in one orbital makes one of the electrons easier to remove
Why does group 3 have a slight dip?
Partial shielding by full s orbital