IMED1004 - Health and Nutrition - Essential Elements (L11, 12)
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
53 Terms
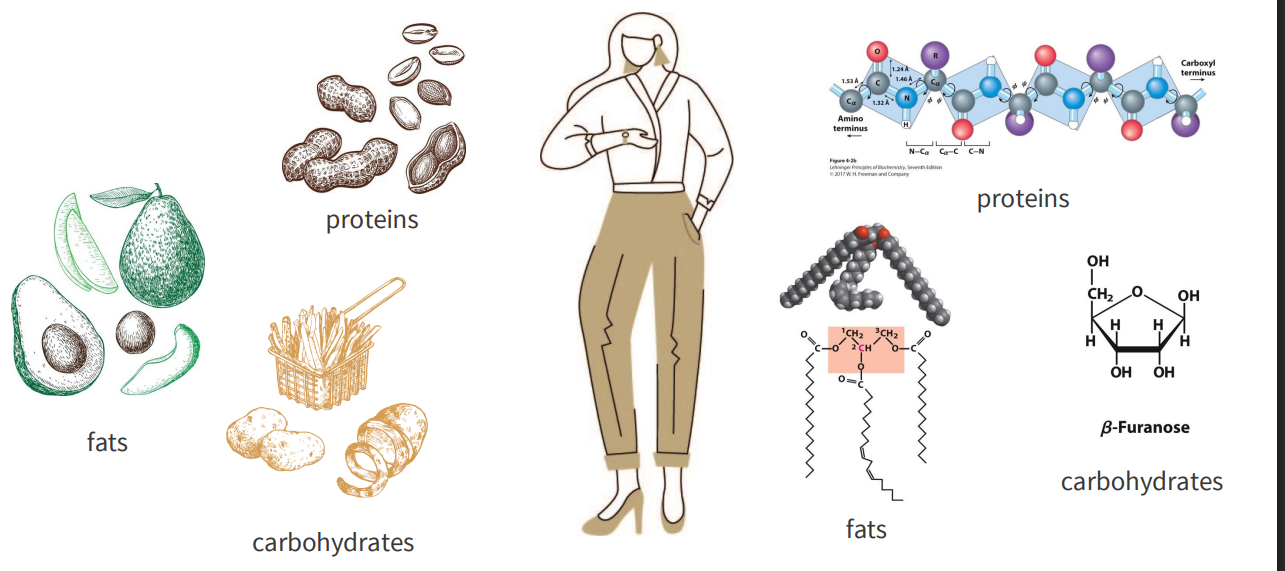
We are made from the foods we eat
DIAGRAM ON SLIDE 5
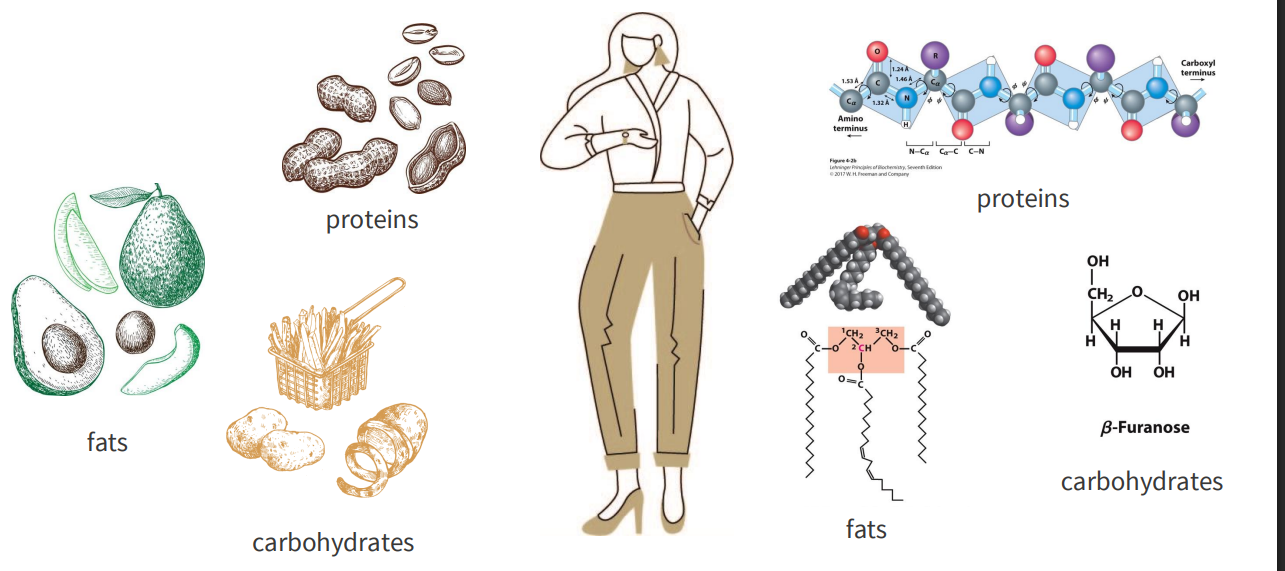
What are nutrients
MACRONUTRIENTS:
- consumed in largest quantities, provide energy
- proteins
- fats
- carbohydrates
.
MICRONUTRIENTS:
- vitamins are organic compoinds (required in small (<1g per day) amounts
- dietary trace minerals (e.g iron) (required in tiny (<100mg/day)
- Required in small quantities for normal physiological function (coenzymes, hormones, structural components)
.
- the nutritional value of food is dictated by what is needed by an organism, and the nutrient content of the food
Causes of Malnutrition
PRIMARY MALNUTRITION:
- Dietary Insufficiency
SECONDARY MALNUTRITION:
- Gastrointestinal (GI) disease
- Chronic wasting diseases
- Acute critical illness, all of which leads to:
- Malabsorption
- Impaired use
- Impaired Storage
- Excessive losses
- Increased requirements
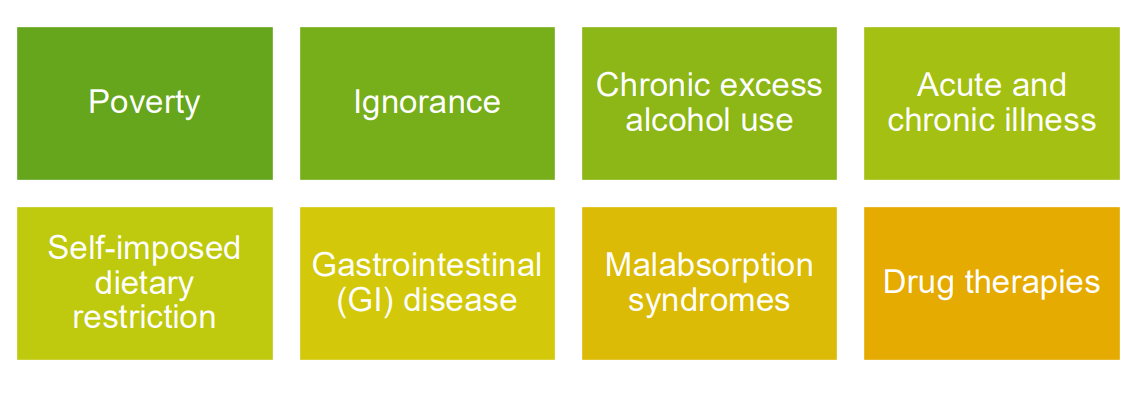
Causes of Malnutrition Listing
- Poverty
- Ignorance
- Chronic excess alcohol use
- Acute and Chronic Illness
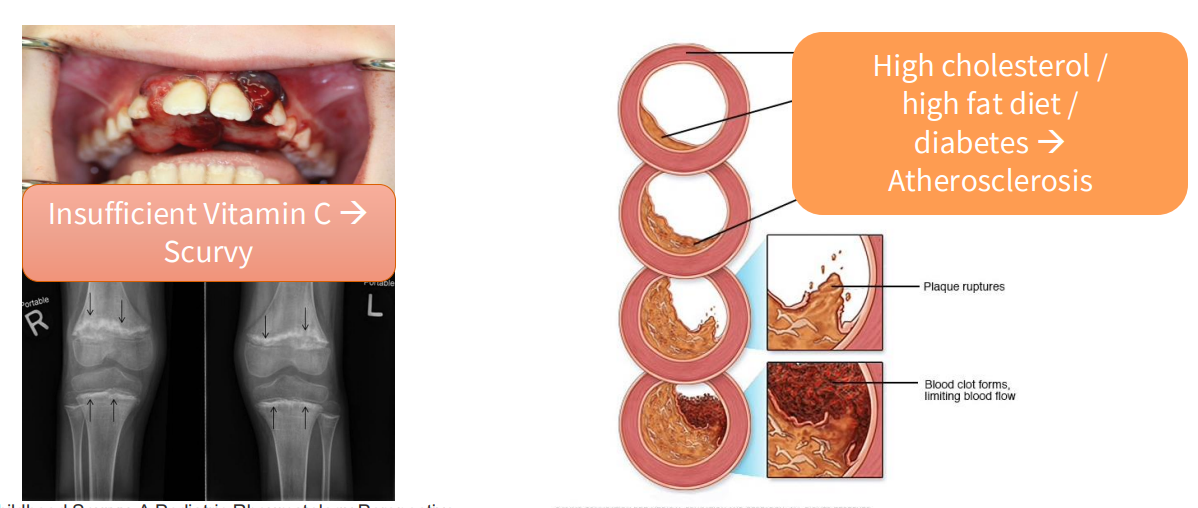
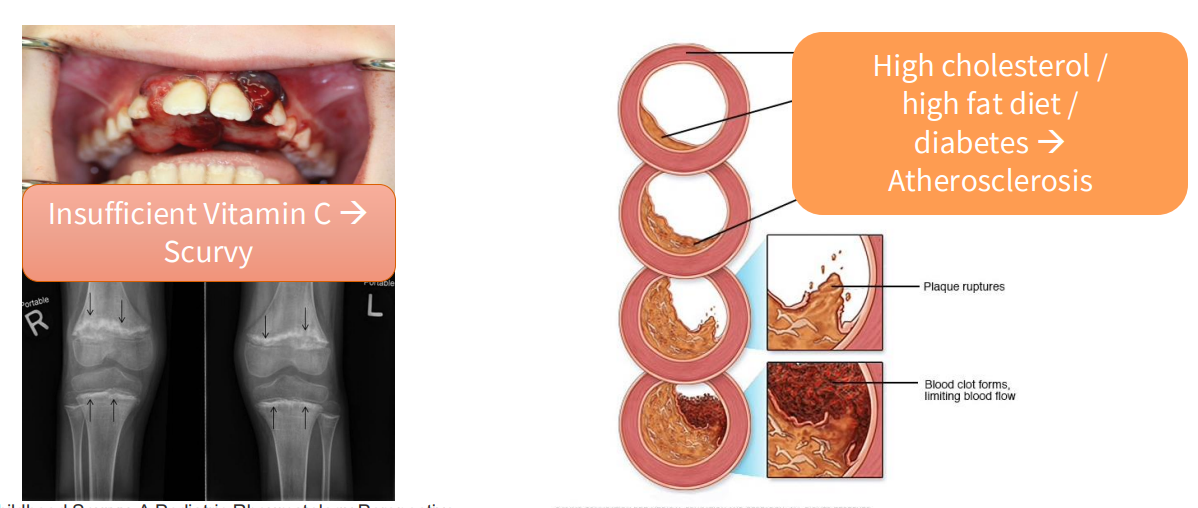
Consequences of Malnutrition
- Insufficient Vitamin C -> Scurvy
- High cholesterol/high fat diet/diabetes -> atherosclerosis
What is nutrition
- Nutrition provides the "building blocks" of biological molecules (Protein -> nitrogen -> nucleic acids)
- What cannot be made endogenously - must be provided exogenously (vitamins, essential amino acids, deficiency in some nutrients can cause morbidity)
- The requirement for energy (carbohydrates, fats, protein catabolism and excess energy intake is a public health problem)
- foods which lead to adverse outcomes (macronutrient/energy imbalance, hypervitaminosis, alcohol)
How do we describe a healthy diet?
NUTRIENT REFERENCE VALUES:
- NHMRC and NZ ministry of Health
- Amount of each nutrient which, on average, meets nutritional needs of most healthy people; and aid in the prevention of chronic disease
- Reference values are specific for age and biological need (childhood, pregnancy, lactation)
- e.g RDI (Recommended Daily Intake)
.
AUSTRALIAN DIETARY GUIDELINES (NHMRC):
- types and amounts of foods, food groups and dietary patterns to: promote health and wellbeing, reduce the risk of diet-related conditions, reduce the risk of chronic disease
- dictated, in part, by Nutrient Reference Values
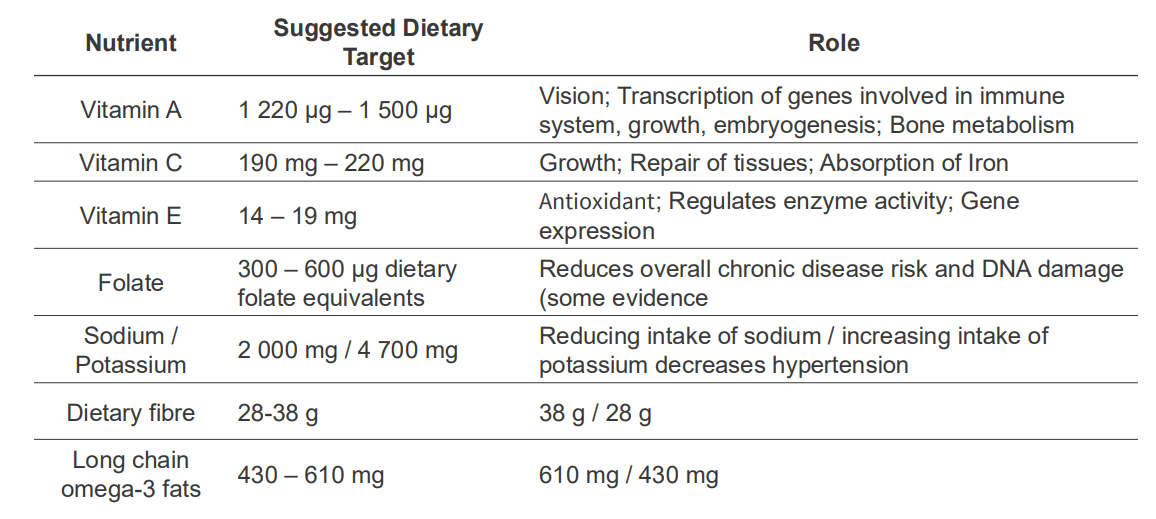
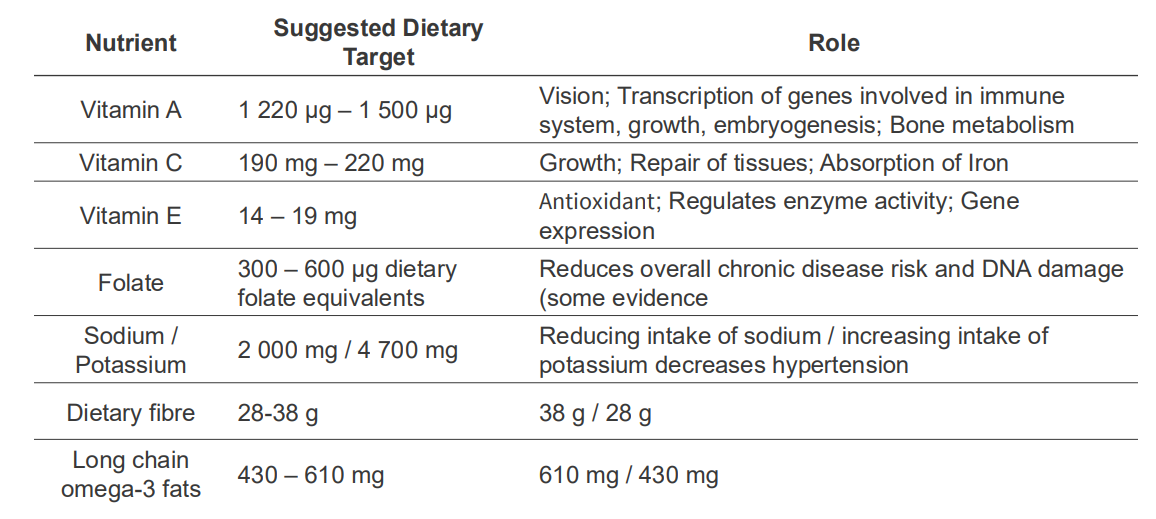
NRVs describe the amounts of nutrients we require, and what we need nutrients for
DIAGRAM ON SLIDE 14

NRVs describe the amounts of nutrients we require and some dietary advice
DIAGRAM ON SLIDE 15

Australian Dietary Guidelines (2013) provide holistic dietary advice
1. to achieve and maintain a healthy weight, be physically active and choose amounts of nutritious food and drinks to meet your energy levels
2. Enjoy a wide range of nutritious foods from the 5 food groups every day:
- Plenty of veggies of different types and colours
- fruits
- grain (cereal) foods, mostly wholegrain and/or high cereal fibre varieties, such as breads, cereals, rice, pasta, noodles, polenta, couscous, oats, quinoa and barley
- lean meats and poultry, fish, eggs, tofu, nuts and seeds, and legumes/beans
- Milk, yoghurt, cheese and/or their alternatives, mostly reduced fat
- plenty of water
.
3. Limit intake of foods containing saturated fat, added salt, added sugars and alcohol
4. Encourage, support and promote breastfeeding
5. Care for your food; prepare and store it safely

Infogrpahic for info from Dietary Guidlines
DIAGRAM ON SLIDE 17

What the lecture will be covering
DIAGRAM ON SLIDE 18
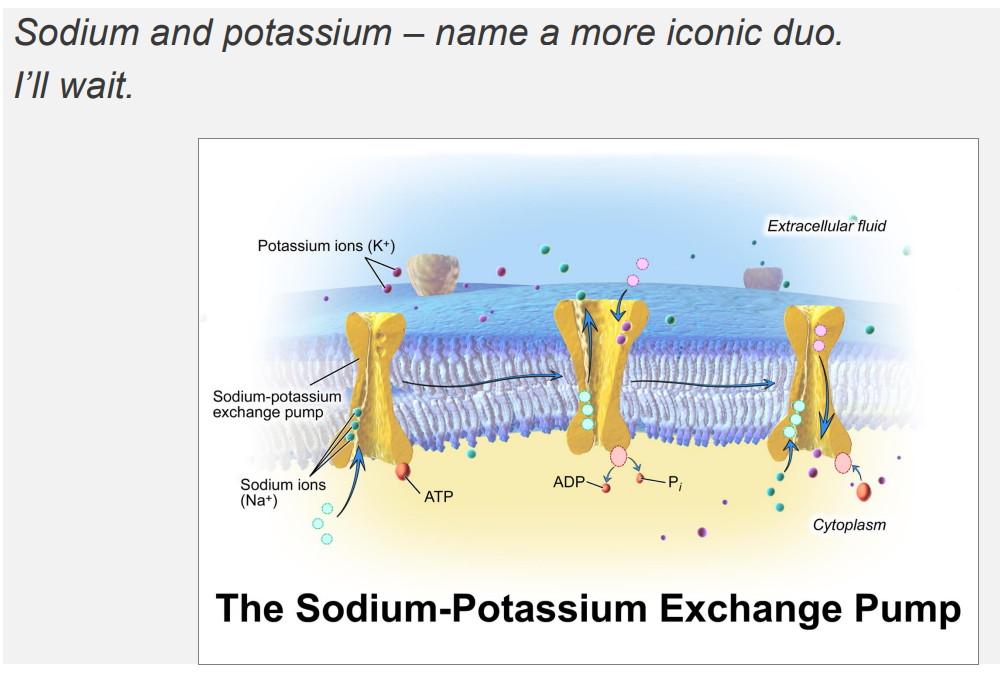
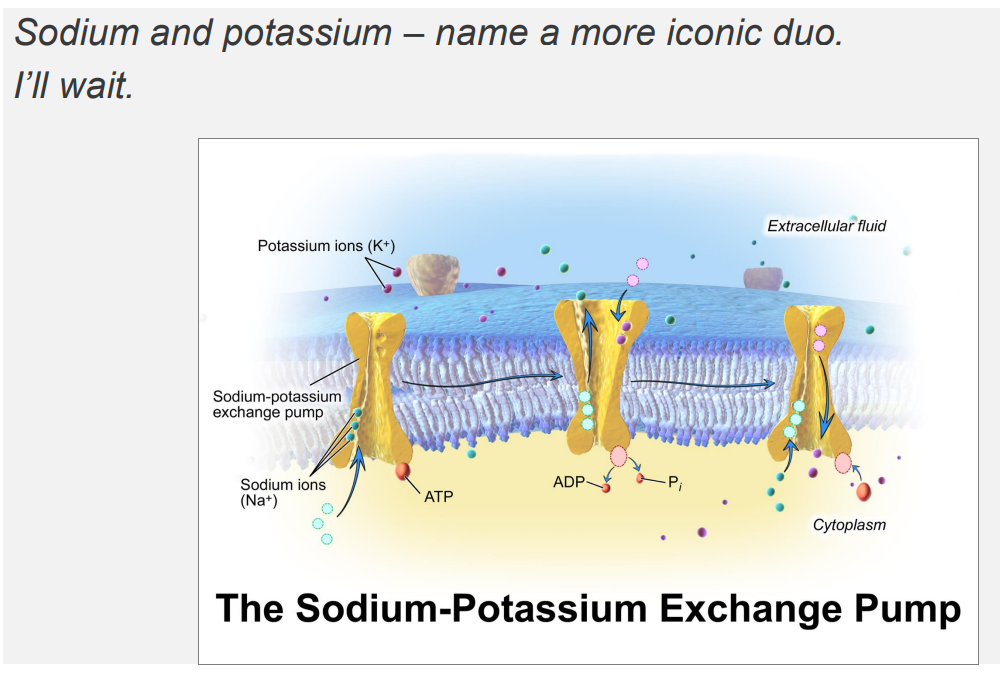
Sodium Potassium Pump DIAGRAM
DIAGRAM ON SLIDE 22
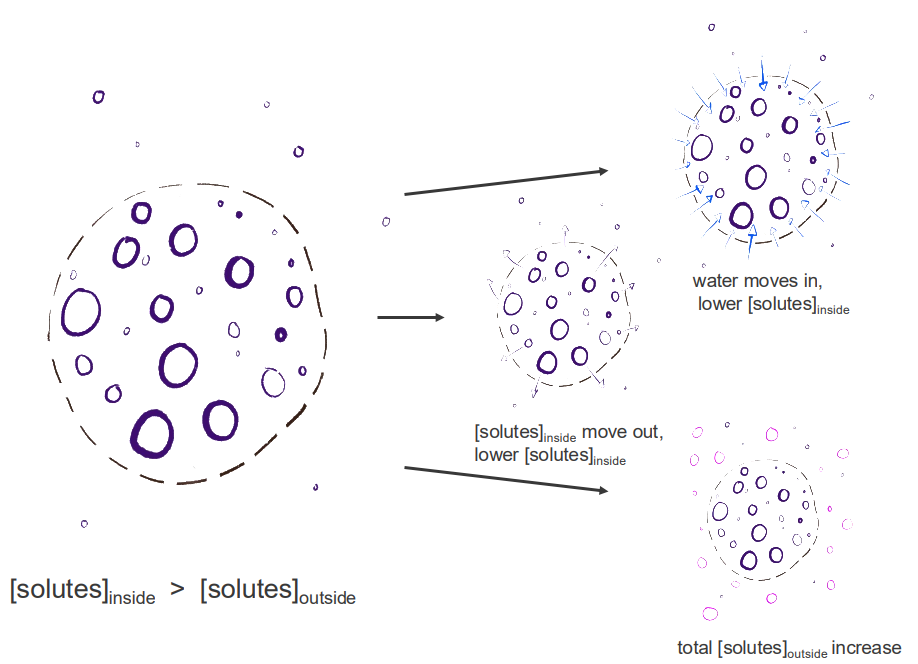
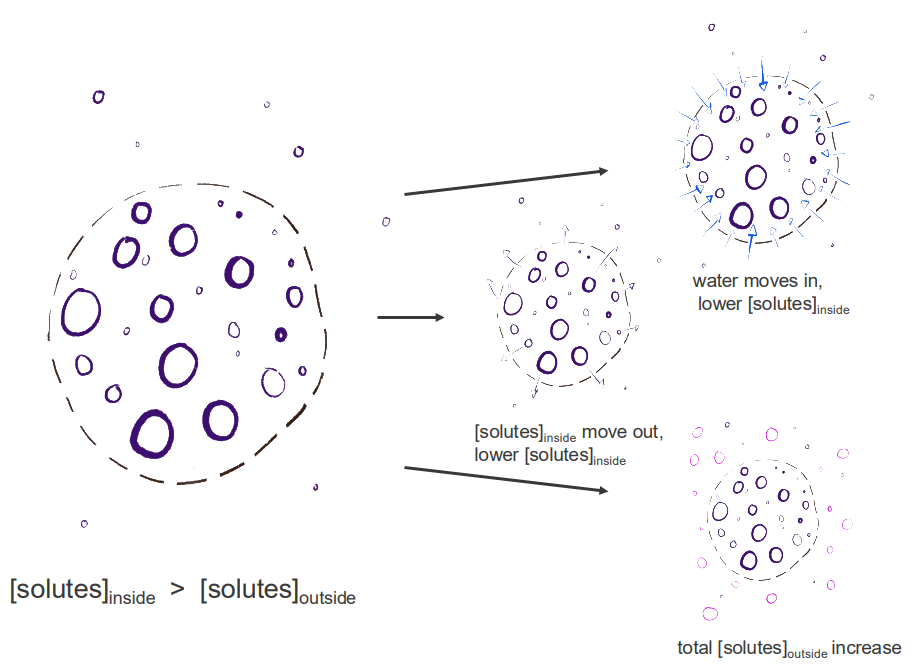
Review: maintaining osmolarity/ormolality
- we have either, depending on the permeability, of the membrane so this dash line represents a semipermeable membrane
- we have gradients, where we have certain amount of solutes inside or outside that structure
- we have movement of water or solutes
- we have solutes moving out maybe
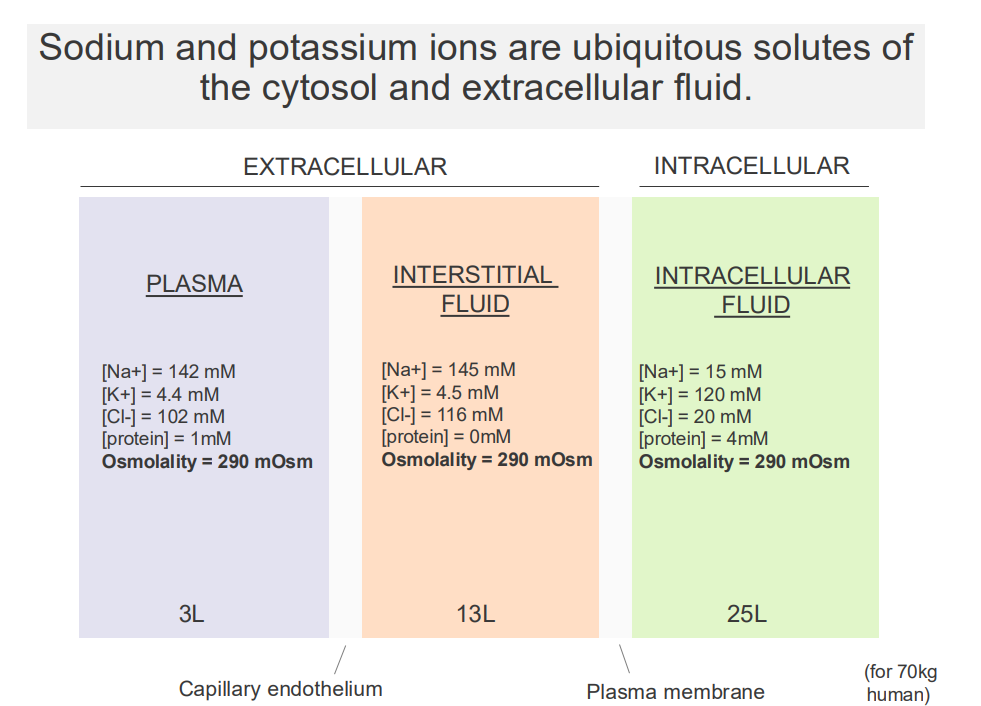
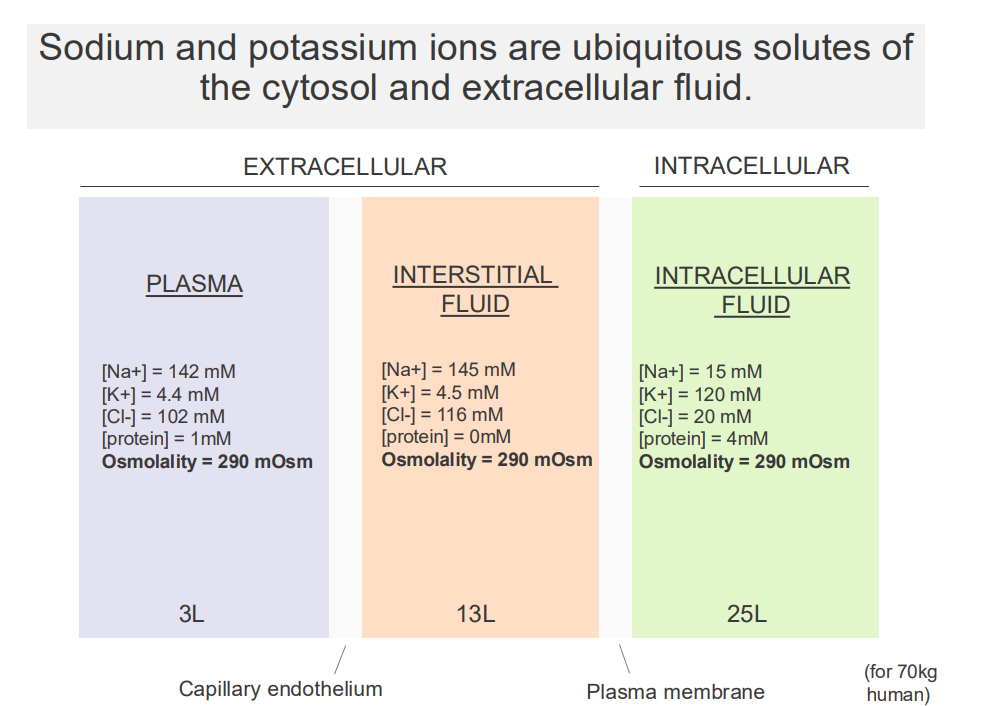
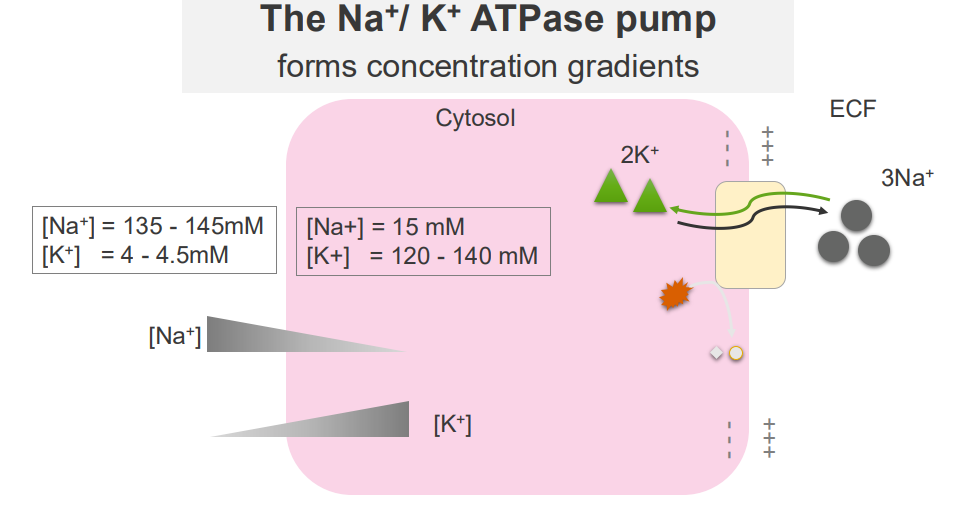
Sodium and Potassium Ions are ubiquitous solutes of the cytosol and extracellular fluid
- major solutes of the ECF and cytosol are Na+ and K+
EXTRACELLULAR:
- Sodium is high in extracellular fluid
- Potassium is low in extracellular fluid
.
INTRACELLULAR FLUID:
- Sodium is low in intracellular, K+ is high
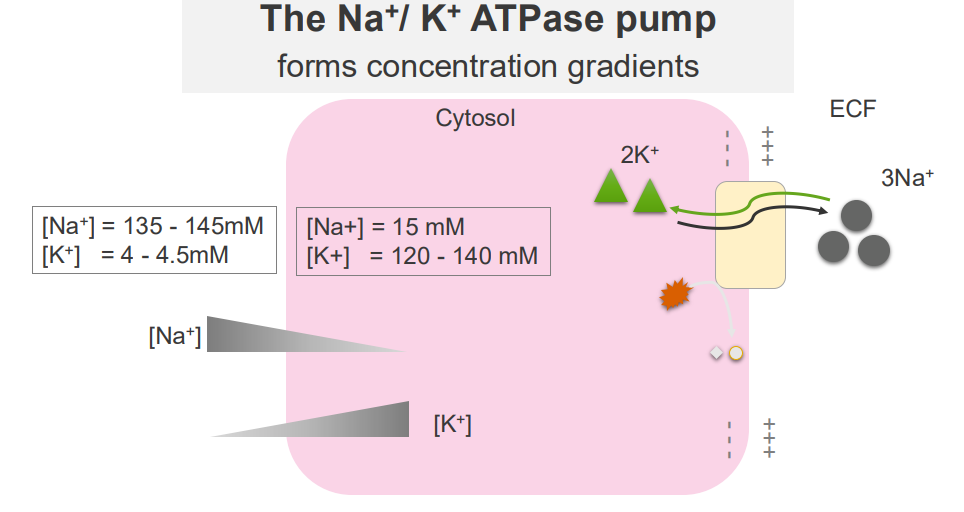
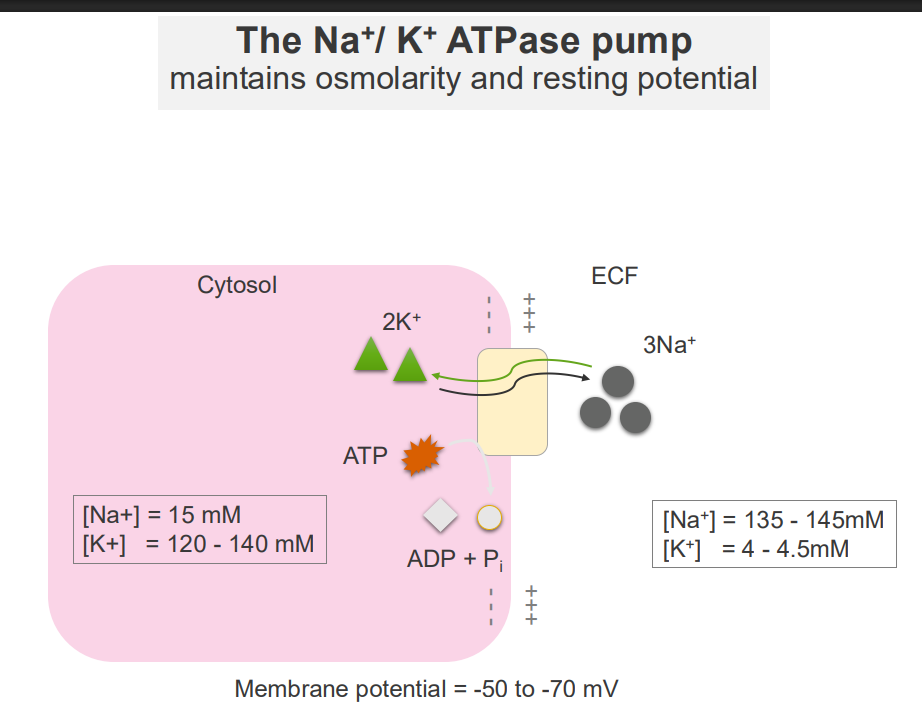
The Na+/K+ ATPase Pump
- it forms concentration gradients
- these concentration gradients facilitate a range of cellular functions: transport of other solutes across the membrane, propogation of action potentials (cell signalling)
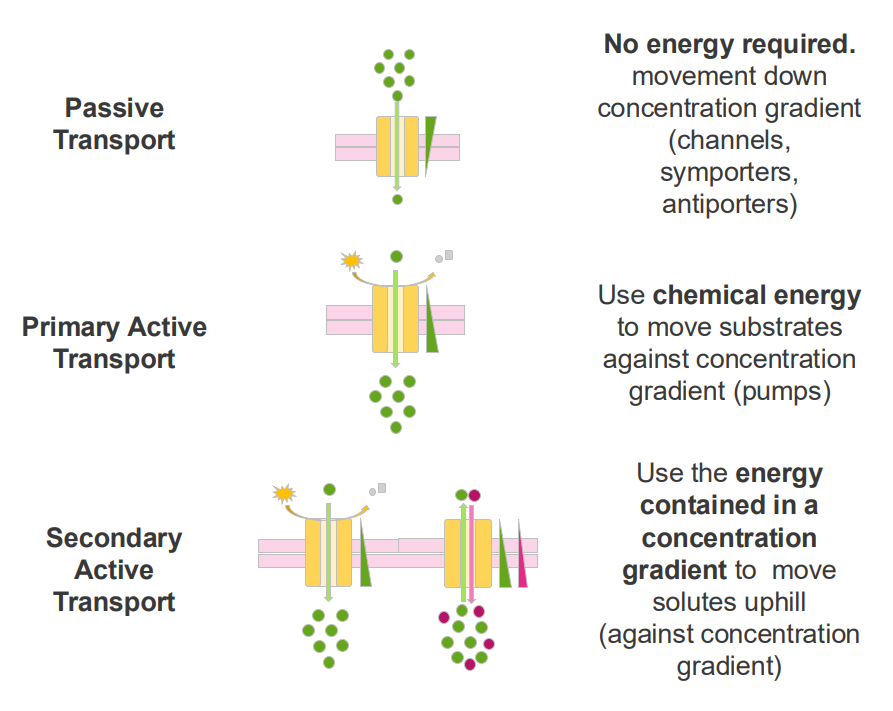
Passive Transport
- no energy required
- movement down concentration gradient (channels, symporters, antiporters)
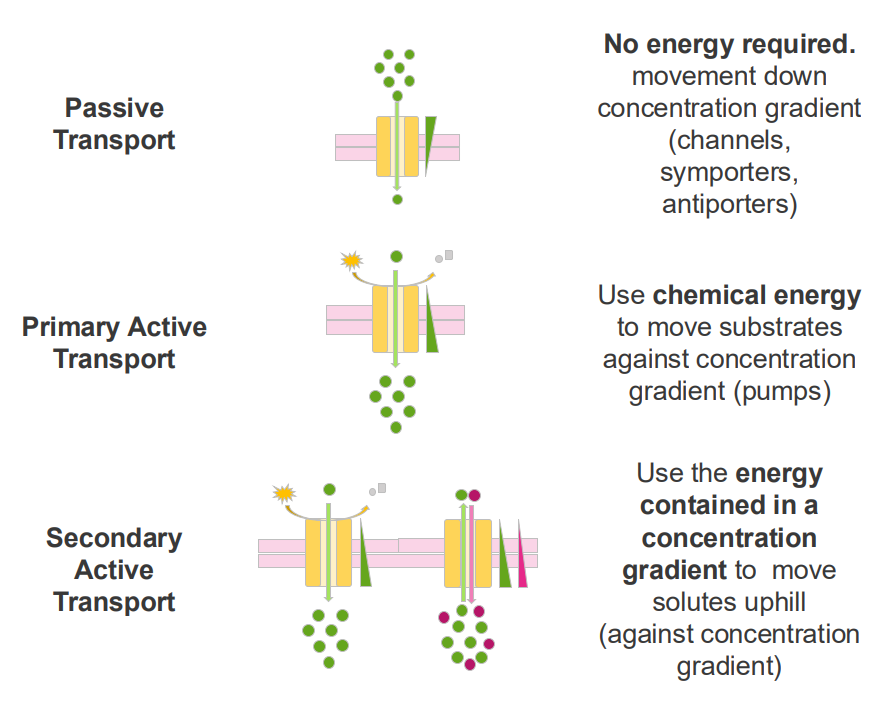
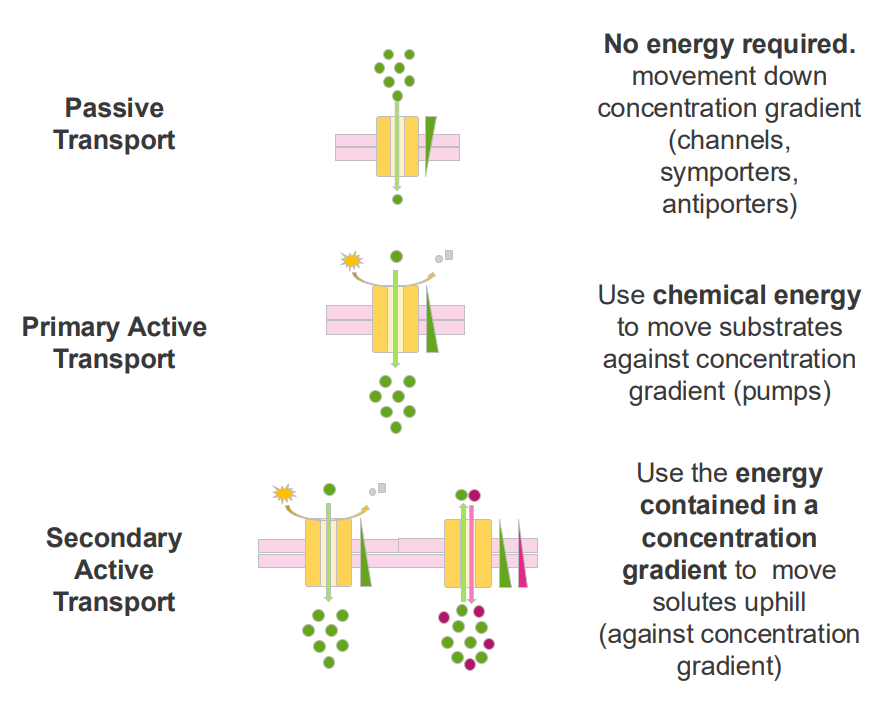
Primary Active Transport
- Use chemical energy to move substrates against concentration gradients (pumps)
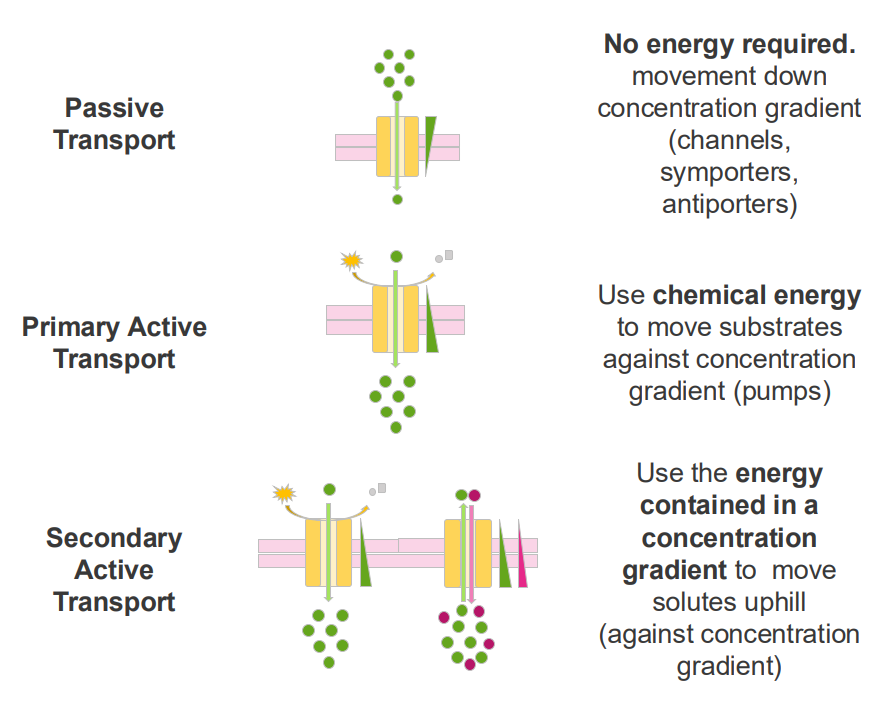
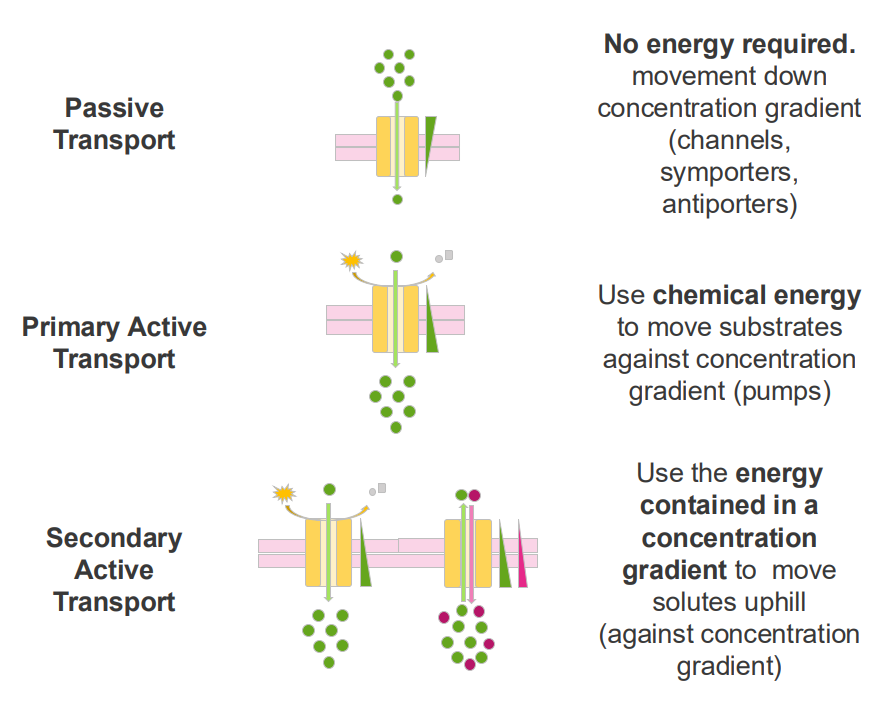
Secondary Active Transport
- use the energy contained in a concentration gradient to move solutes uphill (against concentration gradient)
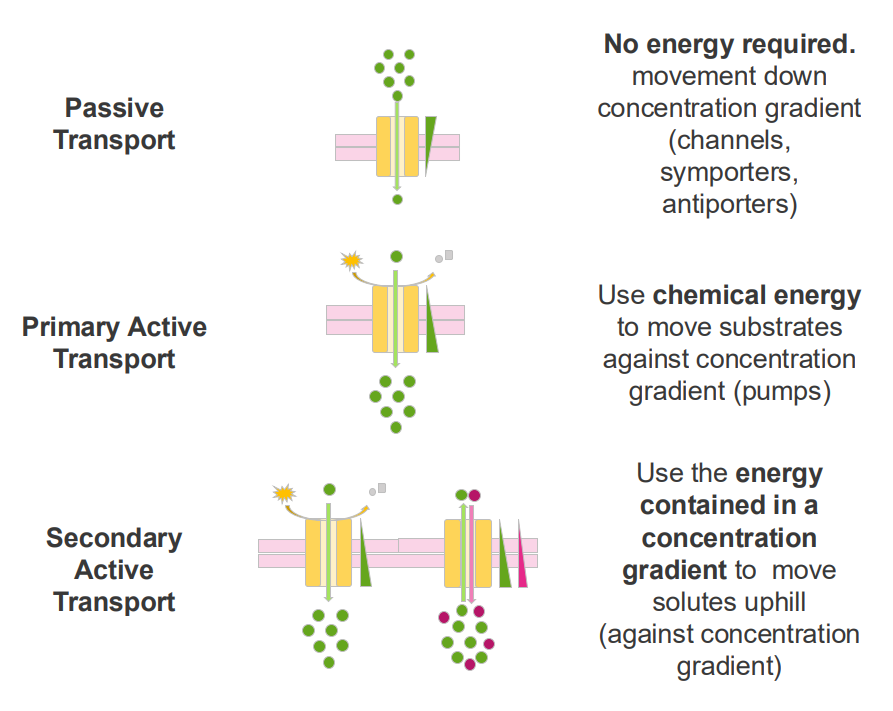
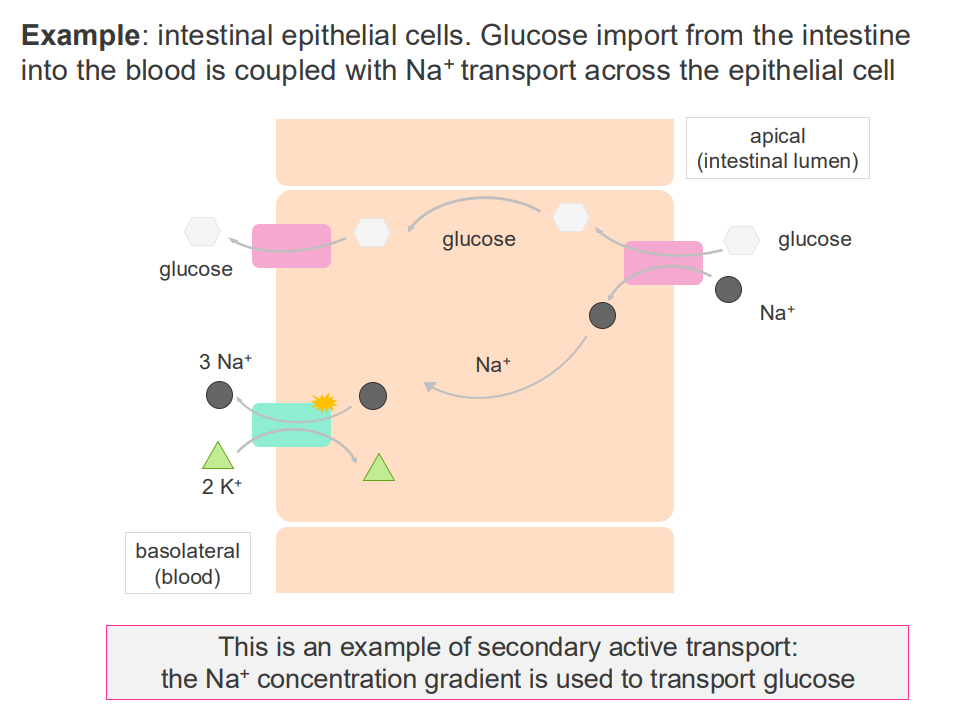
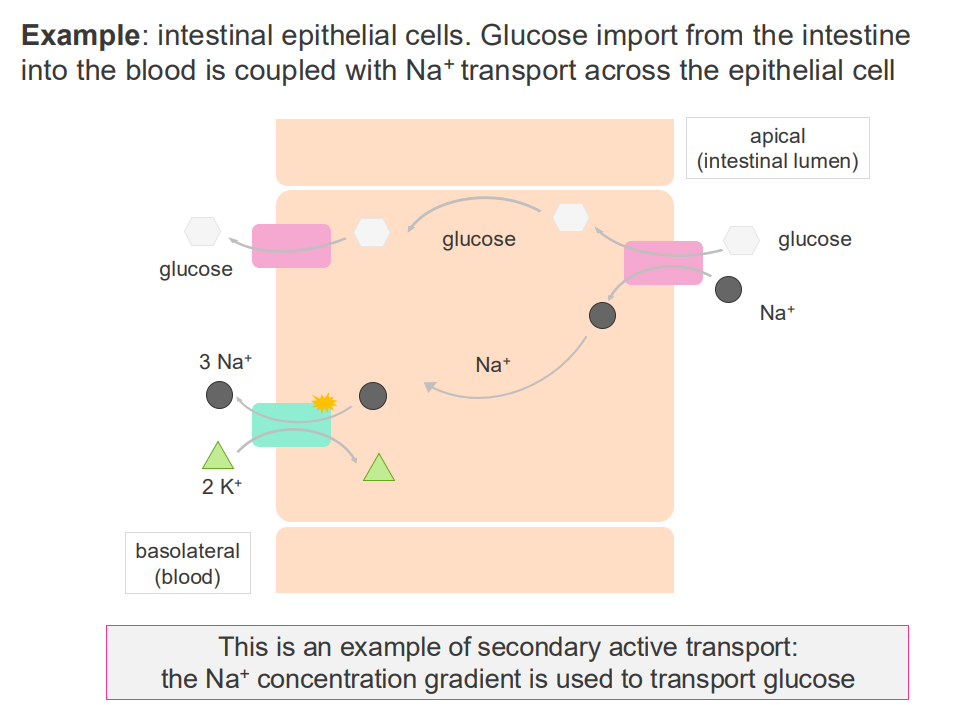
Example of secondary active transport
- interstitial epithelial cells.
- glucose import from the intestine into the blood is coupled with Na+ transport across the epithelial cell
- this is an example of secondary active transport: the Na+ concentration gradient is used to transport glucose
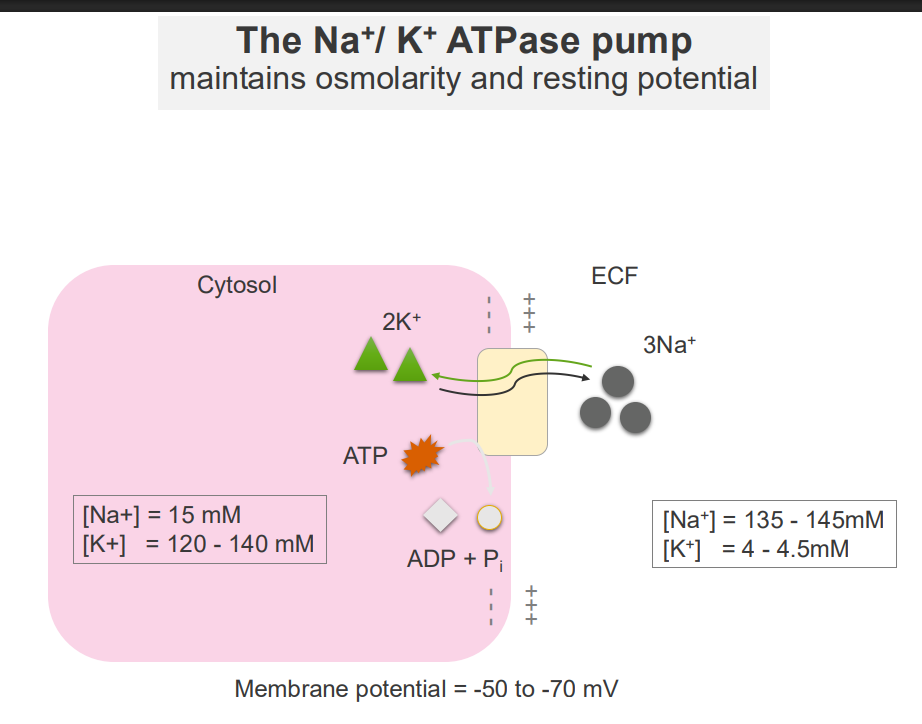
The Na+/K+ ATPase Pump and Osmolarity
- maintains osmolarity and resting potential
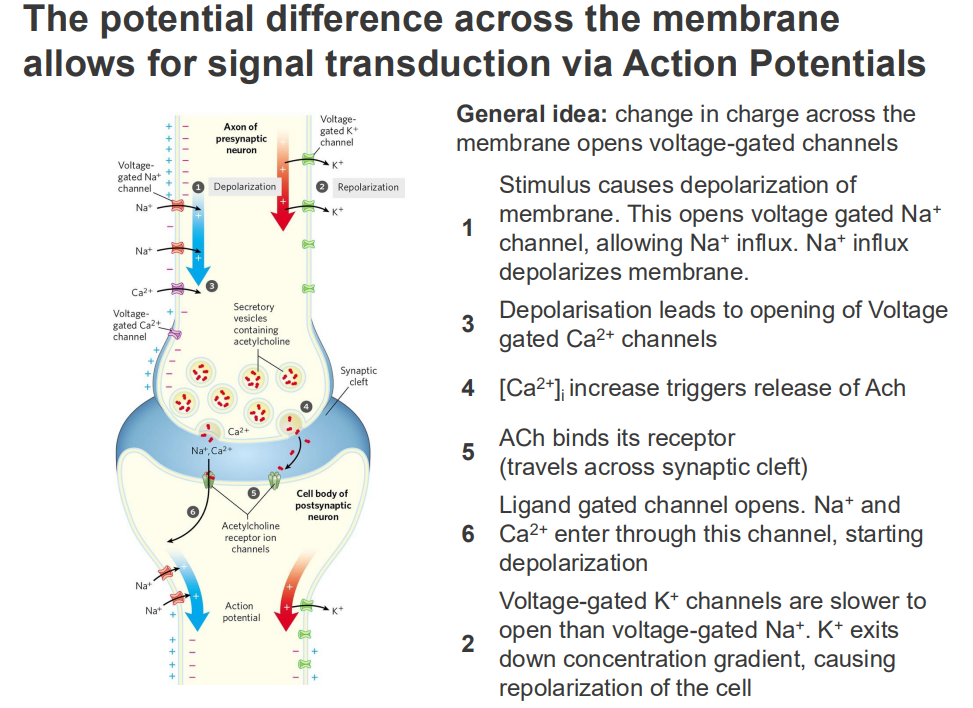
The potential difference across the membrane allows for signal transduction via Action Potentials
GENERAL IDEA: change in charge across the membrane opens voltage-gated channels
1. Stimulus causes depolarisation of membrane. This opens voltage gated Na+ channel, allowing Na+ influx. Na+ influx depolarises membrane
3. Depolarisation leads to opening of opening of voltage gated Ca2+ channels
4. [Ca2+] intracellular increase triggers release of ACh
5. ACh binds its receptor (travels across synpatic cleft)
6. Ligand gated channel opens. Na+ and Ca2+ enter through this channel, starting depolarisation
2. Voltage-gated K+ channels are slower to open then voltage-gated Na+. K+ exits down concentration gradient, causing repolarisation of the cell
![<p>GENERAL IDEA: change in charge across the membrane opens voltage-gated channels</p><p>1. Stimulus causes depolarisation of membrane. This opens voltage gated Na+ channel, allowing Na+ influx. Na+ influx depolarises membrane</p><p>3. Depolarisation leads to opening of opening of voltage gated Ca2+ channels</p><p>4. [Ca2+] intracellular increase triggers release of ACh</p><p>5. ACh binds its receptor (travels across synpatic cleft)</p><p>6. Ligand gated channel opens. Na+ and Ca2+ enter through this channel, starting depolarisation</p><p>2. Voltage-gated K+ channels are slower to open then voltage-gated Na+. K+ exits down concentration gradient, causing repolarisation of the cell</p>](https://knowt-user-attachments.s3.amazonaws.com/8134683d-a9f1-47c6-bc36-a864e4808a0d.png)
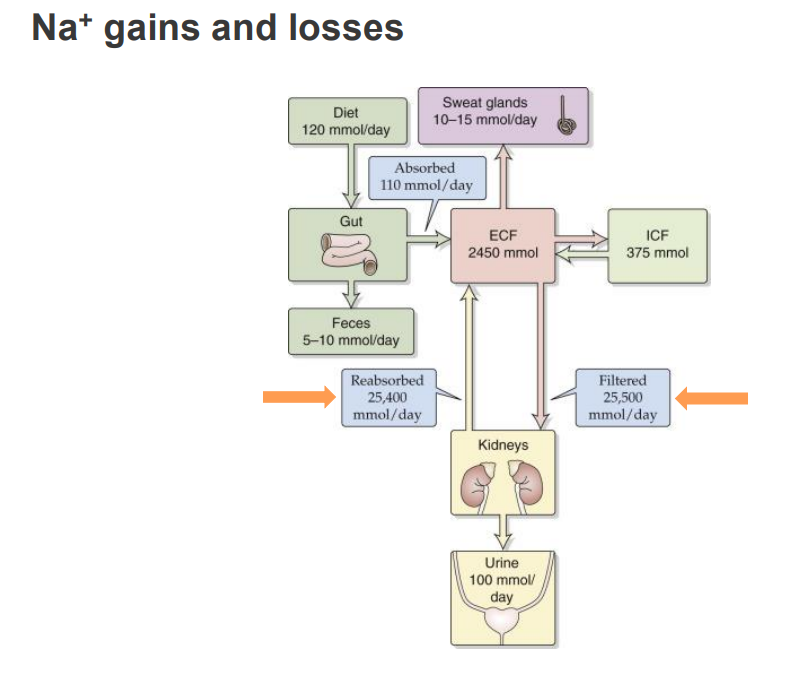
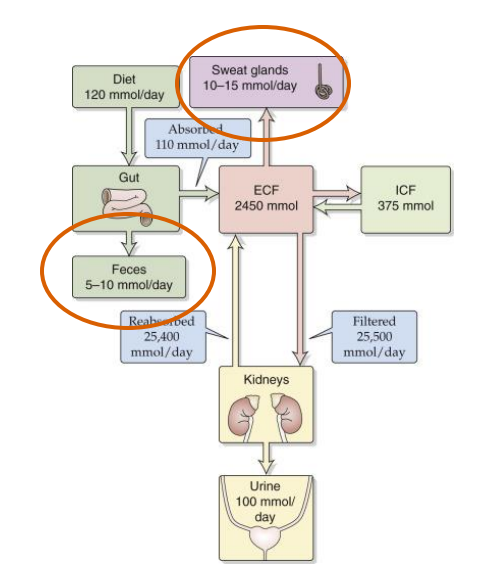
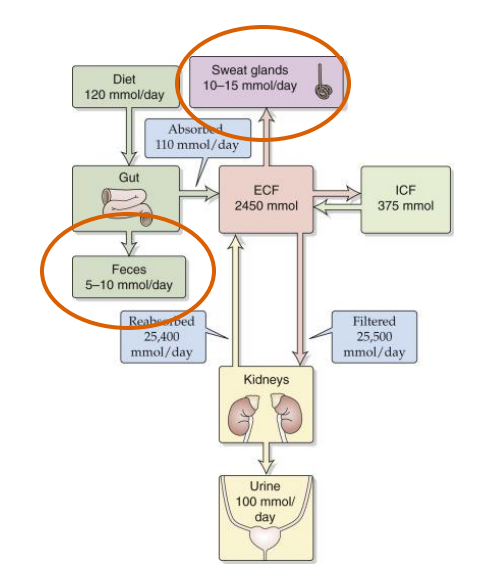
Na+ gains and losses
- with sodium we get loss in the urine
- the diagram shows the ins and outs of sodium
- we get a lot of sodium from our diet
- it comes into the gut and ends up in the extracellular fluid
- and then its filtered by our kidneys where we filter and reabsorb most of it back into our extracellular fluid and that maintains the homeostasis
- its not perfectly reabsorbed, we do get a little loss in our urine but we also lose some in our sweat
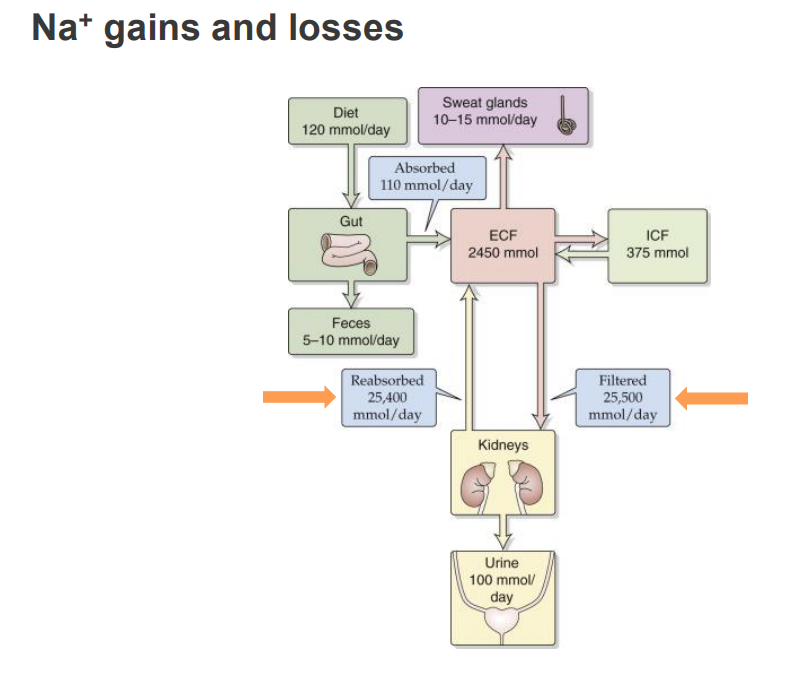
Sodium is oversupplied in the Australian Diet
- the SDT (adults) for sodium is 2000mg/day
- this is lower then the average intake for Australian adults (around 4000mg/day)
- Excessive sodium intake can lead to increased risk of: Hypertension, Heart Attack, Stroke
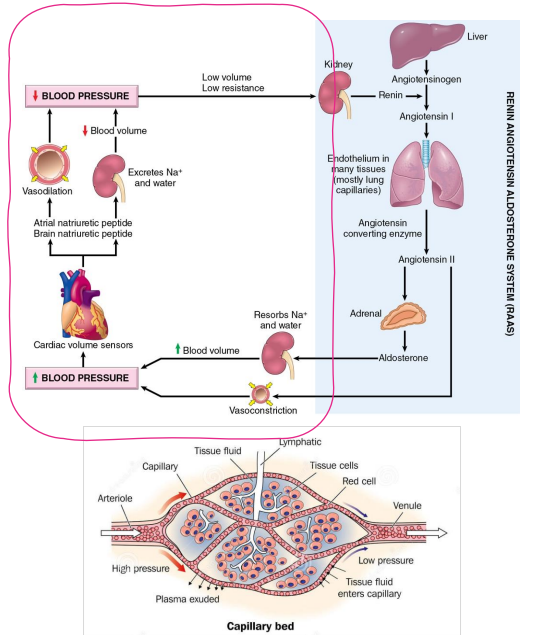
How does Na+ relate to hypertension?
- changes in salt content of the ECF affect ECF Volume
- Extracellular fluid (ECF) volume maintains blood pressure
- Blood pressure is important for adequate tissue perfusion
- circled part of diagram is whats important, not the whole thing
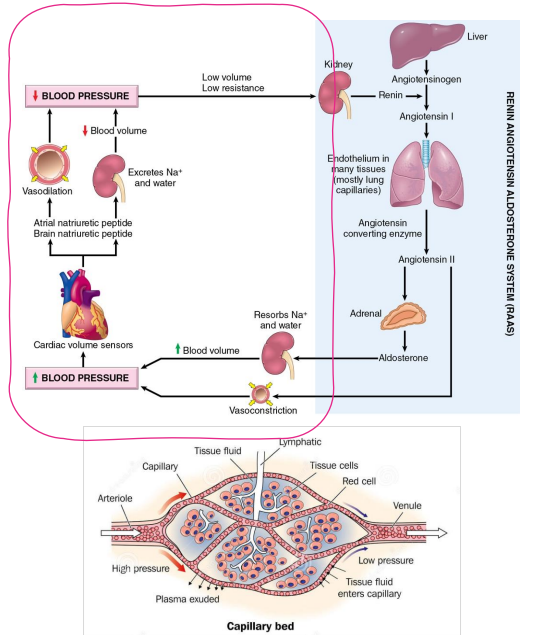
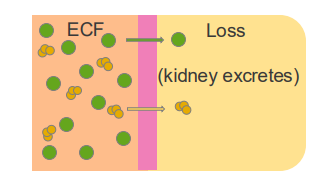
The total-body content of Na+ is the main osmotic constituent of ECF
- Main determinant of ECF Volume
- The total-body content of Na+ is changed through excretion (kidney)
- Initially, decrease in Na+ will change osmolality
- To maintain osmolality, H2O will also be lost
- If the amount of H2O in the ECF is decreased, the volume of the ECF will decrease, leading to a decrease in blood pressure
.
Osmolality measures the number of osmoles of solute per kilogram of solvent (mOsm/kg), while osmolarity measures the number of osmoles of solute per liter of solution (mOsm/L)
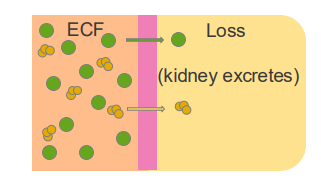
Hyponatremia - Low Na+
CAUSED BY:
- excessive sweating
- vomiting
- diarrhoea
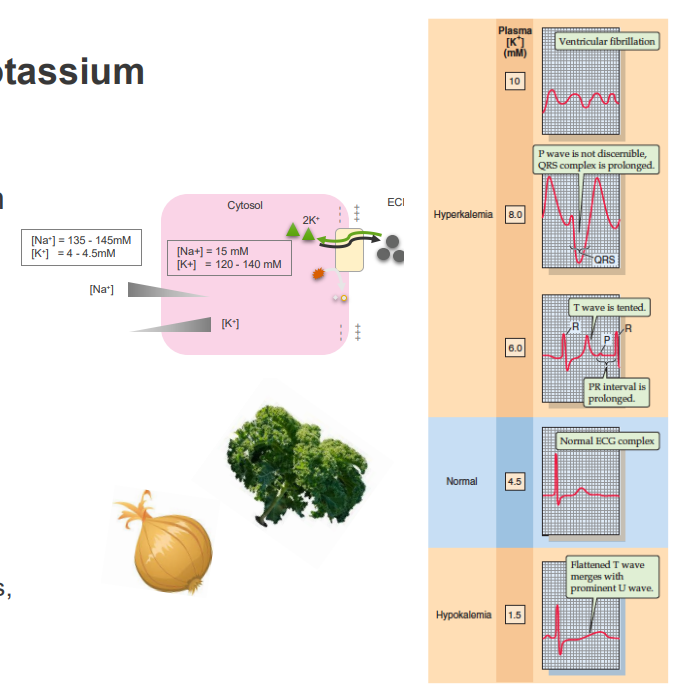
Physiological Roles of Potassium
MAINTAINING CELLULAR POLARISATION (MEMBRANE POTENTIAL):
- Regulation of vascular tone
- Neuromuscular activity
- Cardiac activity
- transport of other solutes
INTRACELLULAR POTASSIUM:
- maintaining cell volume
- regulation of intracellular pH
- control of enzyme function
- DNA and protein synthesis, cell growth
.
Potassium (\(K^{+}\)) is involved in intracellular pH regulation through its exchange with hydrogen ions (\(H^{+}\)) to maintain balance, and through its direct role in the function of ion channels that are sensitive to both \(H^{+}\) and \(K^{+}\) concentrations. During acidosis (\(H^{+}\) influx), \(K^{+}\) moves out of cells to buffer the pH change, while during alkalosis (\(H^{+}\) efflux), \(K^{+}\) moves into cells to compensate. This ion exchange is a critical homeostatic mechanism that helps keep both extracellular and intracellular fluid pH within a normal range.
There is a limit to how much Na+ the kidney can remove. If the amount of Na+ in the ECF increases, what will happen to blood pressure?
- increase in amount of water in ECF
- increase in ECF Volume
- Increase in blood pressure
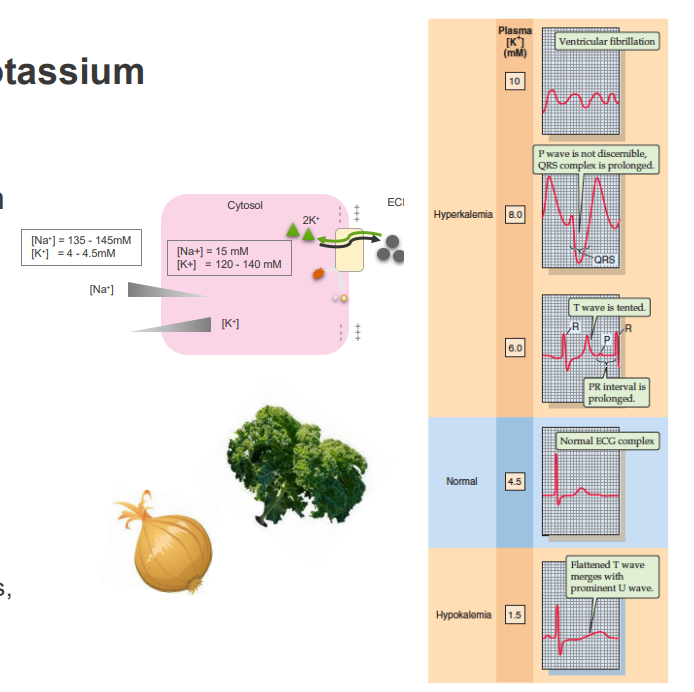
Hypokalaemia (low K+)
- Diuretics, excessive vomiting, diarrhoea
CAUSES:
- extensive burns
- large infusions or intravenous saline
EFFECTS:
- muscle weakness
- heart arrhythmia
POTASSIUM WASTING CAN OCCUR WHERE ECF [NA+] IS HIGH:
- Na+ loss achieved by increased glomerular flow rate
- Adversely affects K+ reabsorption in the kidney
![<p>- Diuretics, excessive vomiting, diarrhoea</p><p>CAUSES:</p><p>- extensive burns</p><p>- large infusions or intravenous saline</p><p>EFFECTS:</p><p>- muscle weakness</p><p>- heart arrhythmia</p><p>POTASSIUM WASTING CAN OCCUR WHERE ECF [NA+] IS HIGH:</p><p>- Na+ loss achieved by increased glomerular flow rate</p><p>- Adversely affects K+ reabsorption in the kidney</p>](https://knowt-user-attachments.s3.amazonaws.com/8e752a85-8fe2-4ce2-b505-e5c496dea320.png)
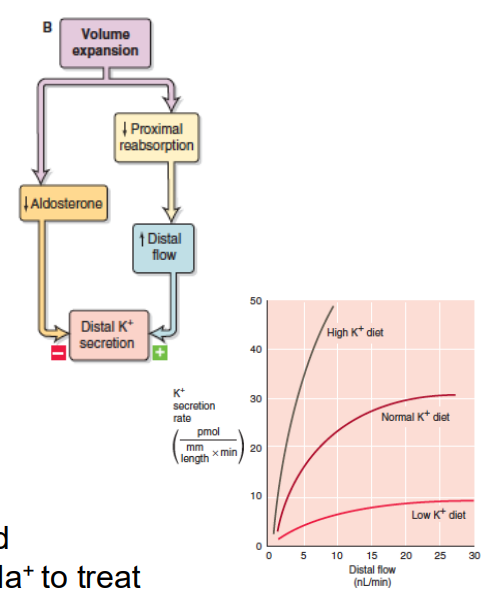
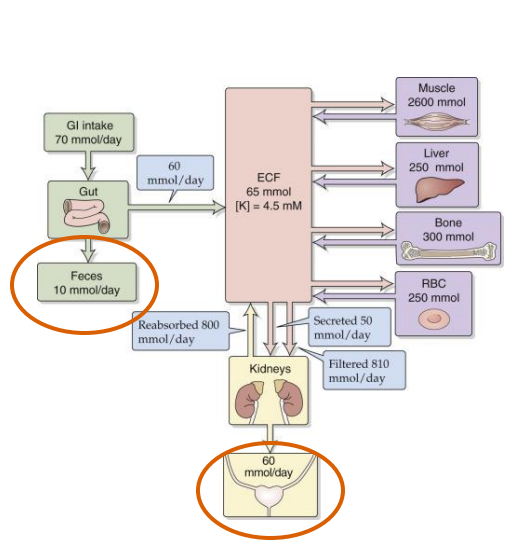
Na+/K+ Balance
- Raised Na+ -> Decreased K+ reabsorption
- Need to consume adequate K+
- K+ has a direct effect on vascular tone
- Hypokalemia -> exacerbation of the primary hypertension
- For this reason, the dietary guidelines recommend considering increasing K+ intake when reducing Na+ to treat sodium sensitive hypertension
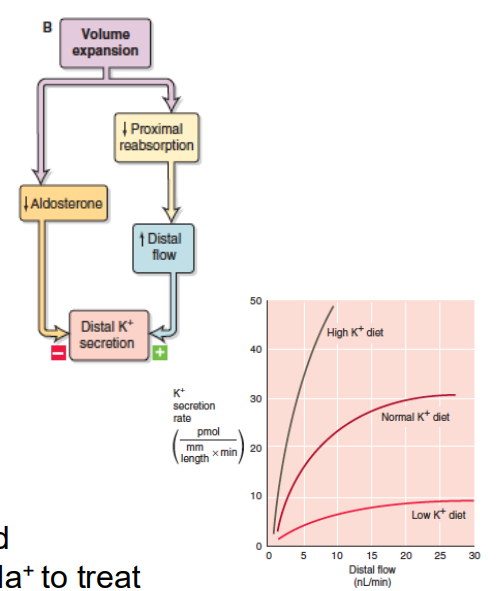
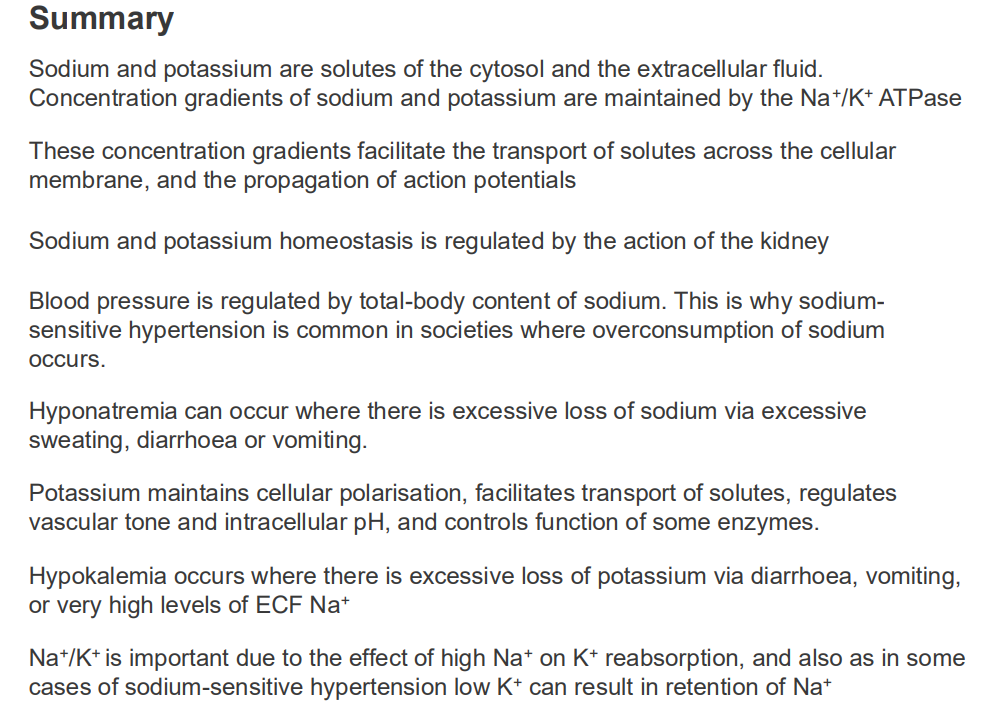
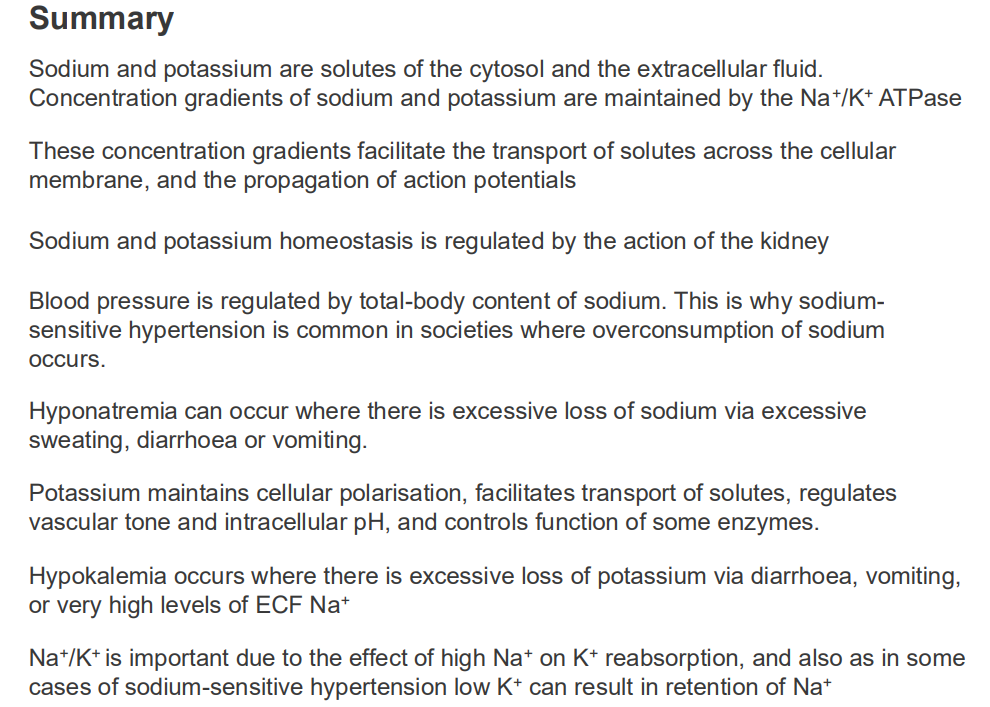
SUMMARY SO FAR:
DIAGRAM ON SLIDE 39
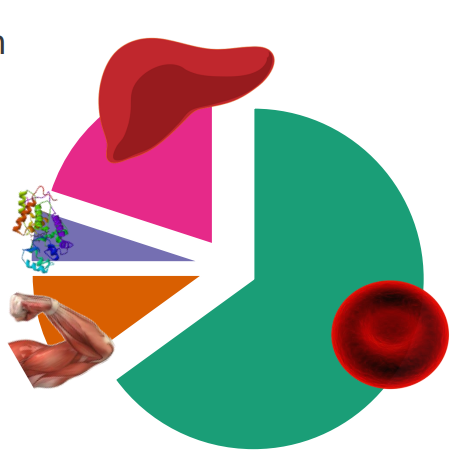
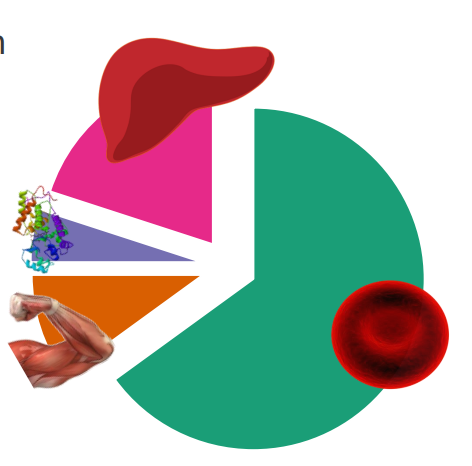
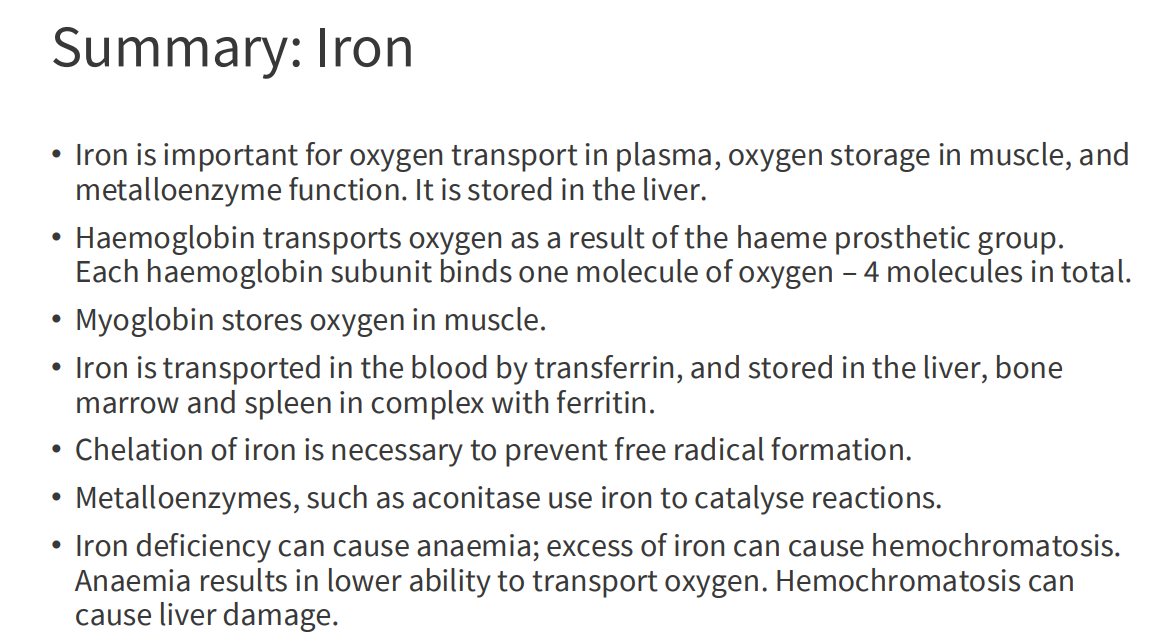
Iron in the human body
- iron is localised in a range of tissues to perform relevant functions
- human body has around 2-4g of iron (38-50mg iron/kg body weight)
- 65% is haemoglobin
- 10% myoglobin
- 1-5% is metalloenzymes
- 20% in storage
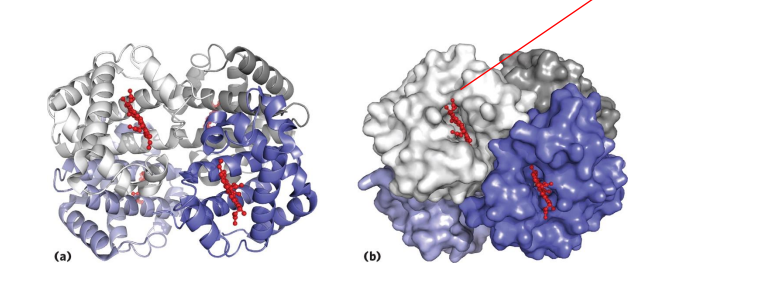
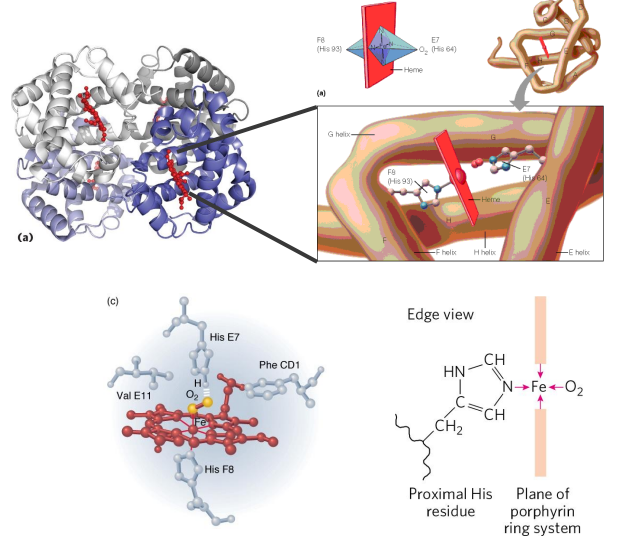
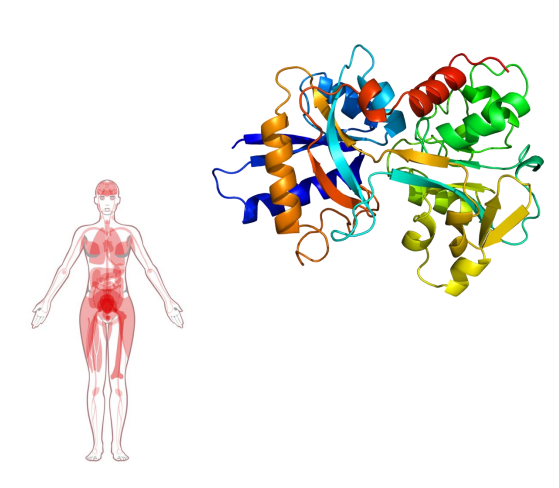
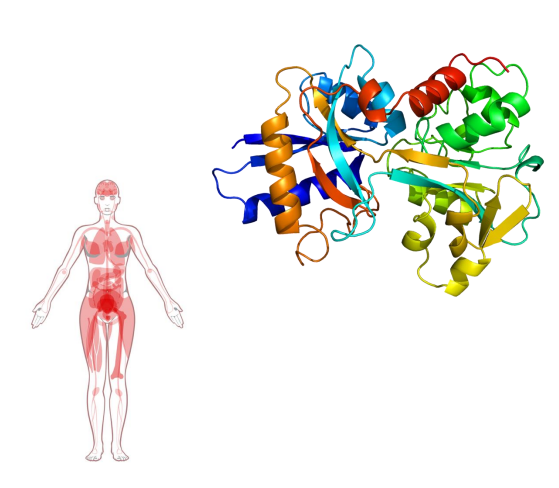
Haemoglobin
- contains a haem (of heme) prosthetic group (in red in diagram)
- haemoglobin has 4 subunits
- each subunit contains one haem
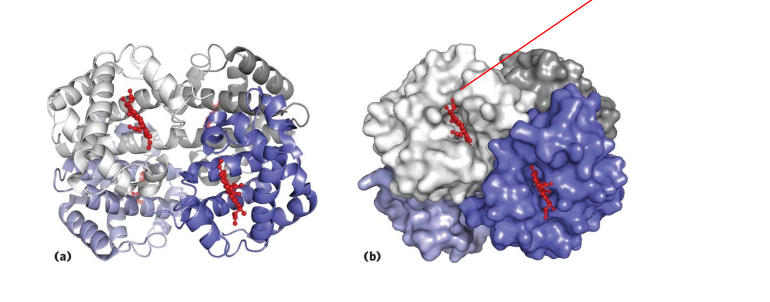
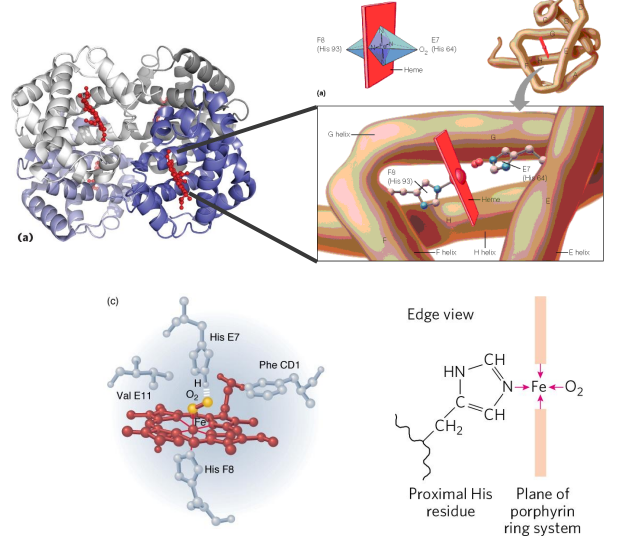
Haemoglobin Function
- O2 is not soluble in aqueous solutions
- the haeme prosthetic group binds O2 and is carried in the blood
- Each subunit can bind one molecule of O2
- Cooperative binding: binding of O2 to one subunit increases affinity of the other subunits for O2. Binds where O2 is high, releases where O2 is low
.
- in the diagram we can see that oxygen is binding to the iron (the yellow dot in the diagram is iron)
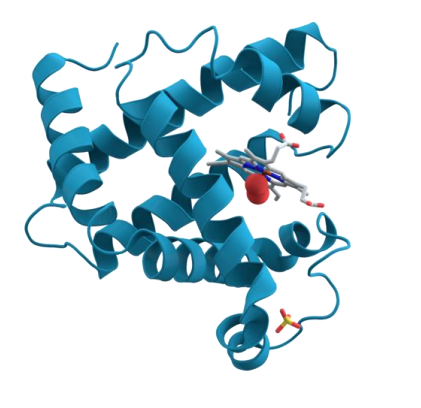
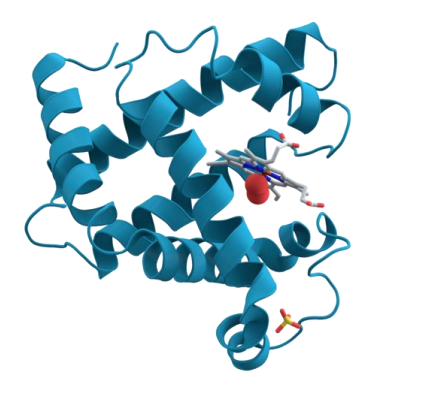
Myoglobin
- contains one haeme group - also binds O2 (stronger affinity for O2 then haemoglobin. Harder to release)
- 1 subunit
- only found in muscle
- also binds oxygen
- function - oxygen storage in molecule
.
- myo means muscle which means its only found in muscle
- this allows us to store oxygen in our muscle
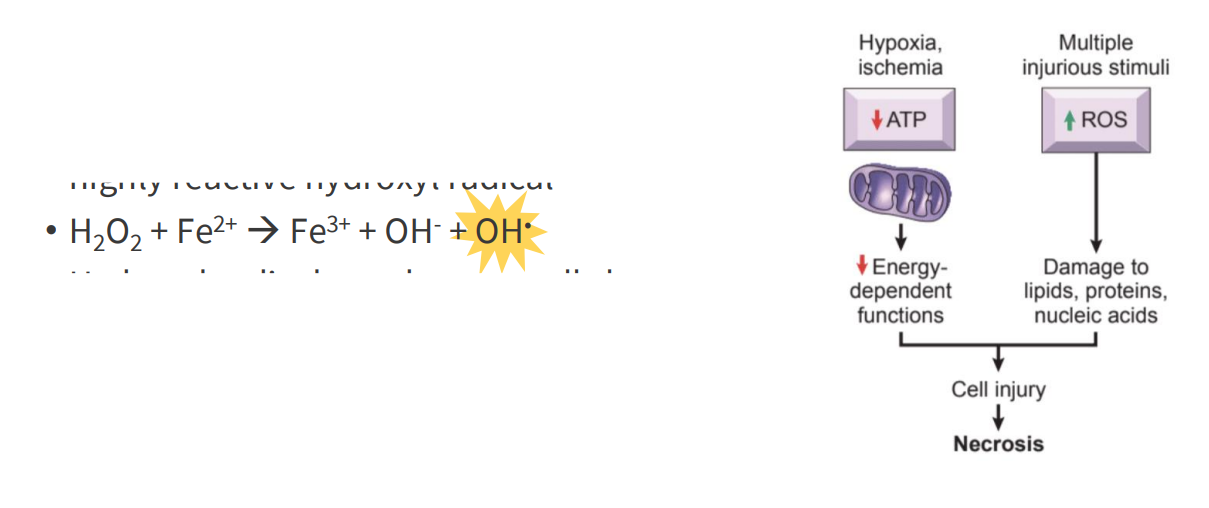
Iron and Free Radical Formation
- two oxidation states: Fe2+ (ferrous) and Fe3+ (ferric)
- Fe2+ readily converts Fe3+, produces the highly reactive hydroxyl radical
- H2O2 + Fe2+ -> Fe3+ + OH- + OH. (the last OH is shining)
- the OH radical is formed (the shining OH). this is highly reactive
- hydroxyl radical can damage cells by oxidising biomolecules
- Ferritin and transferrin proteins bind iron, preventing oxidative damage
.
- basically we have to be careful about how we transport and store iron becuase if we have a lot of ferrous iron (Fe2+) in the same compartments where we have H2O2 being produced, we end up with free radical which could damage biomolecules and result in cell injury
Iron Transport
- Transported in the blood by transferrin
- Bound to transferrin in the Fe3+ (ferric) state
- Cells contain transferrin receptors
- Once inside the cell, released from transferrin and reduced to Fe2+ (ferrous) state (stored in ferritin, incorporated into haem)
.
- measure saturation of transferrin
- iron deficiency -> lots of unbound transferrin
.
- diagram shows transferrin, and where its being expressed in the body
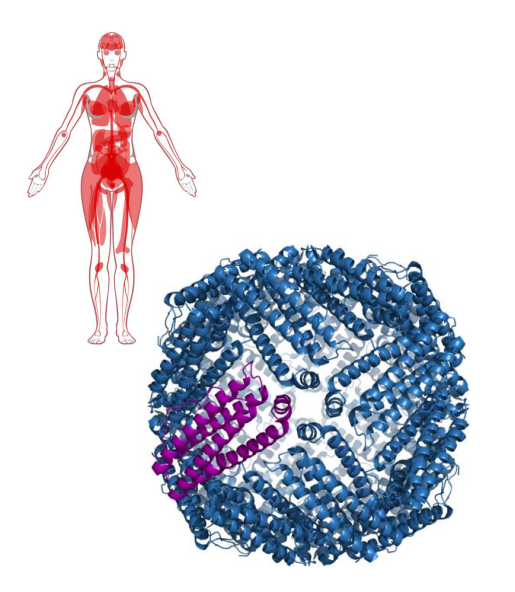
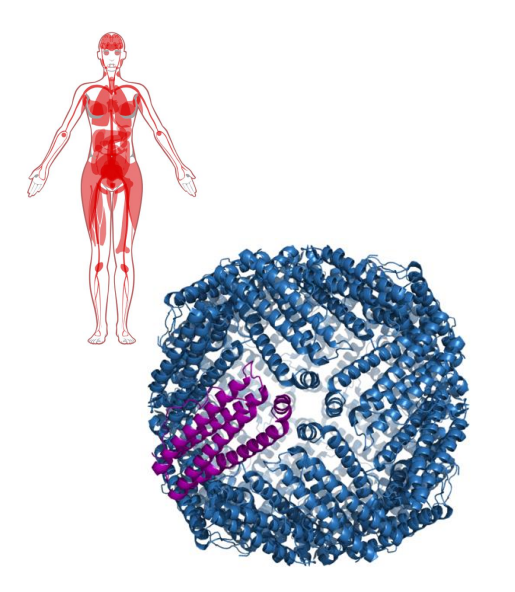
Iron Storage
- storage sites: liver, bone marrow and spleen
- Iron forms a complex with ferritin
- Ferritin is in Cytosolic form in almost all cells to prevent formation of free radicals (we need to chealate iron (sequester it))
- 24 subunit protein, forms a nanocage
- Stores 4500 Fe2+ ions
.
- diagram shows ferritin
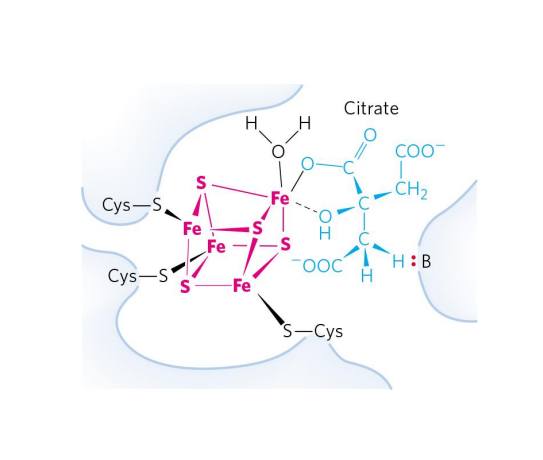
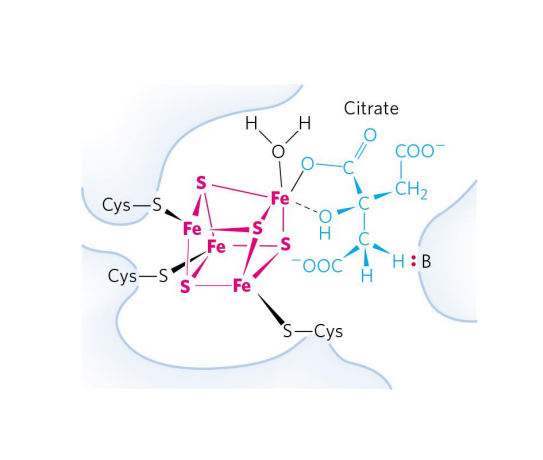
Metalloenzymes
EXAMPLE:
- Aconitase (TCA Cycle)
- Converts citrate to isocitrate
- Fe-S centre interacts with the substrate (citrate) to catalyse the reaction
.
- this is a learning outcome so u need to know this slide well

Iron Intake
- higher intake required for individuals who lose blood by menstruation
- Haem iron from animal flesh is more bioavailable
- Non-haem iron from plant sources: Absorption is aided by ascorbate (Vitamin C)
- Foods with high levels of iron: lentils, beans, nuts, leafy greens, fortified grains, dried fruit, meat

Iron Deficiency
- Most common nutritional deficiency in the world
- Iron deficiency anaemia - haeme lacks Fe2+
- Insufficient O2 Delivery to tissues
.
CAUSES:
- inadequate nutrition (infants have an increased requirement, however breast milk is not rich in iron)
- Slow, chronic bleeding (commonly gastrointestinal)
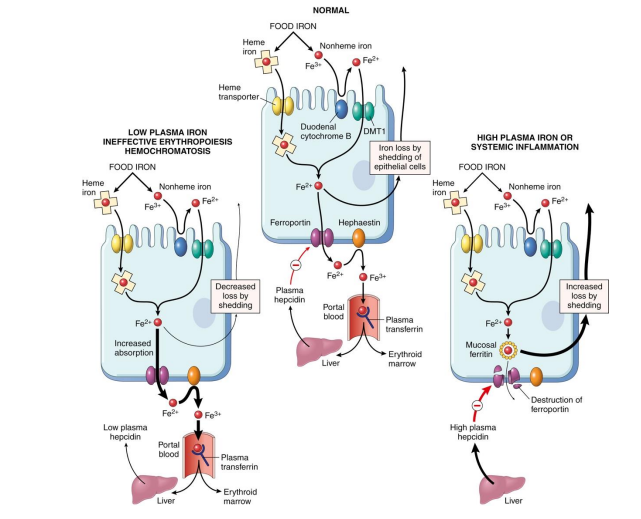
Disruptions to Regulation of Iron Absorption in Haemochromatosis, Inflammation
- NO LEARNING OUTCOME, DESPITE WHAT THE SLIDE SAYS. SHE HAS CHANGED IT
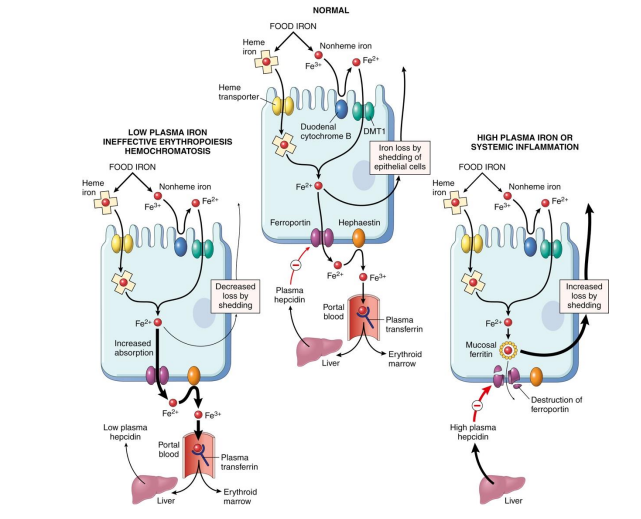
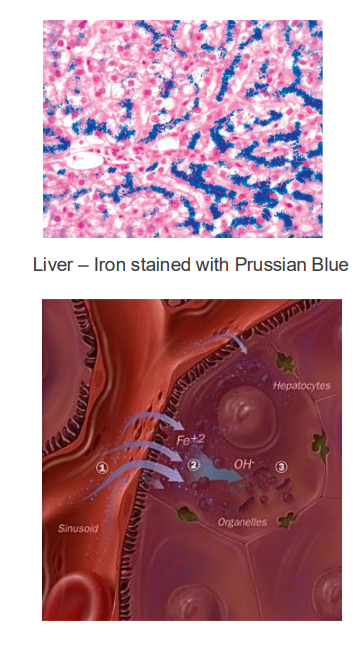
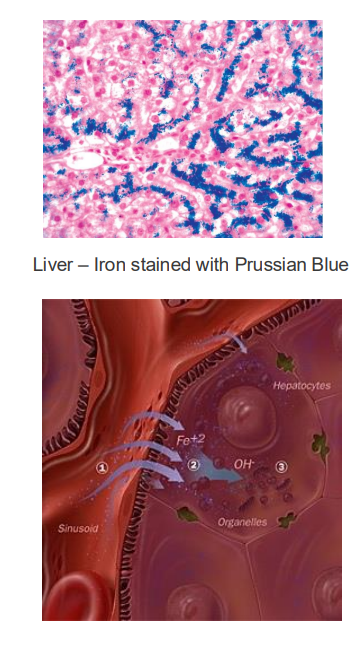
Iron Excess
EXCESS:
- haemochromatosis (hereditary, genetic disease)
- iron builds up: deposits in liver, heart and joins
- Can lead to cirrhosis (scarring) of liver
.
TREATMENT:
- give blood regularly
- vegetarian diet (foods less dense in iron)
.
- basically what happens is that if u have the disease, you lose the signal that tells the endothelial cells to stop uptaking iron when iron is sufficiently high. We'll have an inappropriate amount of iron being transported into our bodies. And this is a problem because if we're having this buildup of iron, obviously we'll try and do things that sequester iron, maybe make a bit more ferritin, try make sure it doesnt react with H2O2 and produce superoxide molecules. But there will be a limit to how much we can do if someone keeps eating a lot of iron containing foods, and so we can end up with deposits in the liver. this iron produces reactive oxygen species which damages tissues
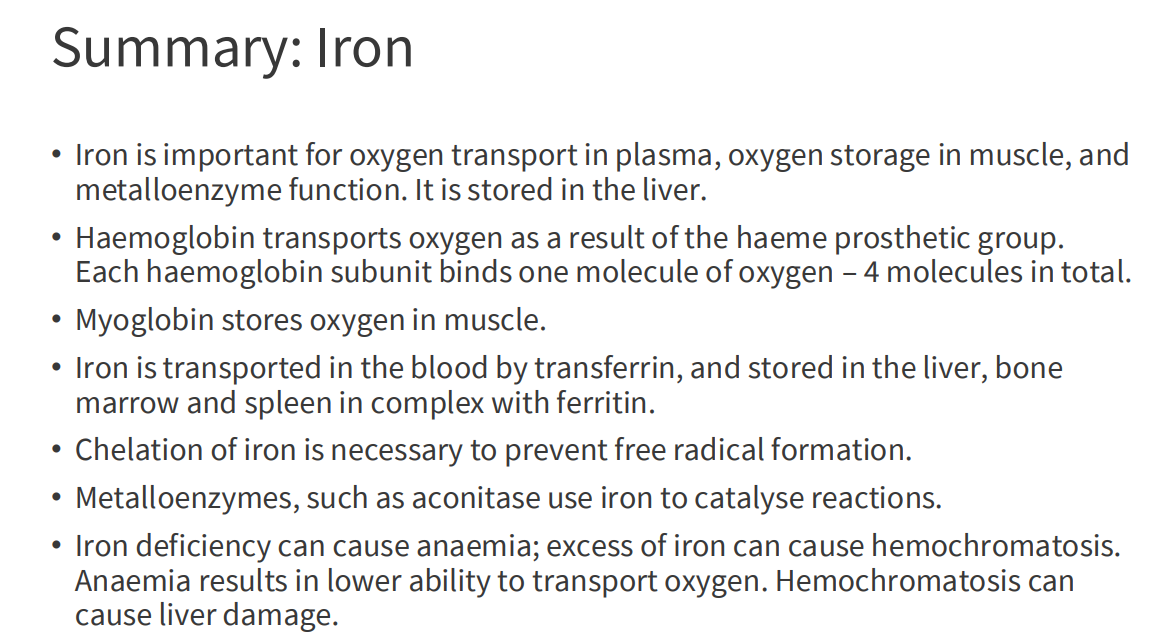
SUMMARY OF IRON:
DIAGRAM ON SLIDE 55
Calcium
- Role in Signalling (neuromuscular and cardiac)
- Muscle contraction is triggered by Ca2+
- involved in neuron firing
- Signalling for apoptosis
- 99% is in bone (skeleton)
.
- we constantly change levels of Calcium in bone to adjust levels. if we need more calcium in blood we steal it from the bones
Calcium Excess
- Calcium has an UL (upper limit) (risk of kidney stones) - 2500mg
- if we have too much it increases risk of kidney stones
Calcium Deficiency
- Osteoporosis, loss of bone density
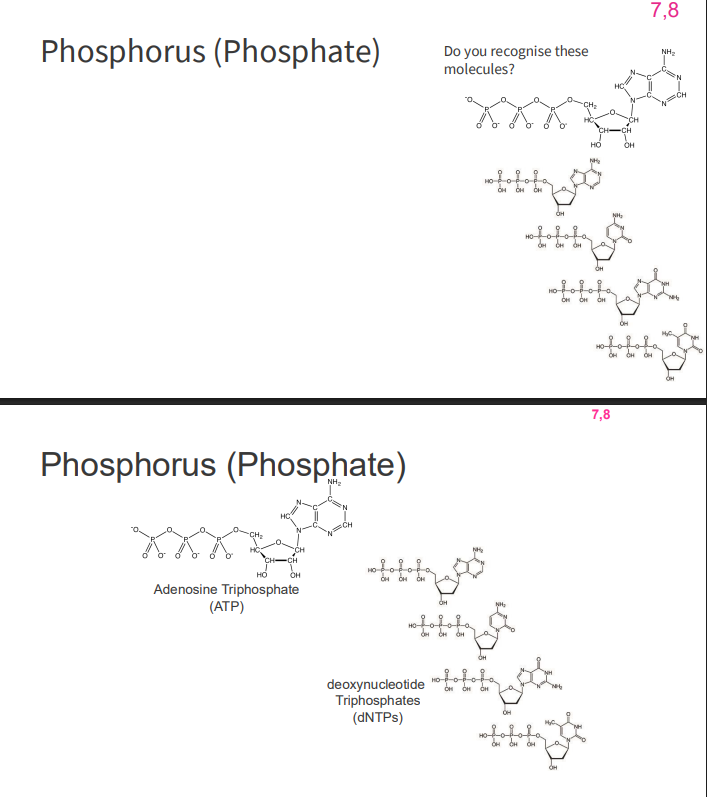
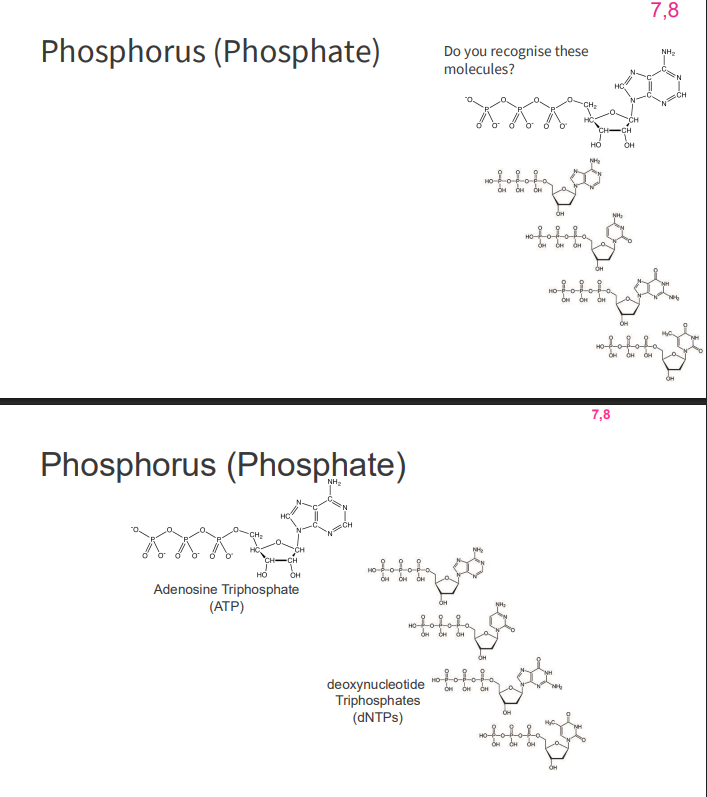
Phosphorus (Phosphate)
- second most abundant inorganic element in our body (behind oxygen)
- role in chemicals which provide cellular energy (ATP, GTP)
- role in nucleic acids (dNTP -> dNDP)
- GTP also has a signalling role
Phosphorus (Phosphate) Deficiency
- Hypophosphatemia is rare - very well supplied in diet
SYMPTOMS:
- anaemia
- muscle weakness
- rickets
- ataxia
- confusion
- death
Magnesium
- Cofactor of more then 300 enzymatic reactions
- Mg-ATP 2- complex is the active form of ATP
- DNA and RNA synthesis
- Protein synthesis
- Metabolism
- Many other processes
.
DEFICIENCY is rare
- muscle spasm
- nausea
- muscular weakness
Iodine
- One of the first trace elements to be identified as essential
- Now supplemented in salt - public health intervention
- Component of thyroid hormone T3 and T4 (growth, development of CNS, energy production, oxygen consumption)
Iodine Deficiency
- congenital deformity (severe neurological issues: deafness, mental deficiency)
- goitre, hypothyroidism