Cardio last exam... pray for me and my people
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
98 Terms
What statistic does hypertension affect poeple in the united states. what is teh result of untreated hypertension
it affecrs ½ of people over 18 and 2/3 of people above 65
it is the leading cause of chronic kidney disease, heart attack, and stroke. does not discriminate agains ancenstry, reaces and genders
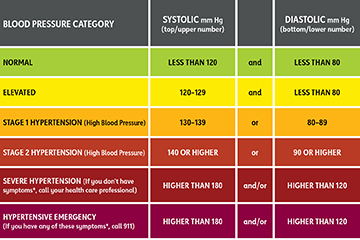
Describe the trends based on the hypertenison chart
konw what causes each
What is the root of the hyepertension issue: essential hypertension/ genrereal population/raas)
essential hypertension (90-95%) of cases
no primary/ identifiable cause
General populaiont
typically begins with hypervolimea (increased sodium and water retention
this shifts the pressure naturesis curve= higher arteiriol pressure is needed to maintain sodium blanace
Enhanced raas pathway activity
What is the ma equation
Co x svr
Waht is essential hypertension adnd how is it diagnoesd/ what causes it
Diagnosi by exclusion
vascular changes contribute to yperstensive states = hypertrophy oer time
it is related to heredity, age, race, socioeconomic status, sexual orientaion, sex, and gender
some patients with essential hupertension are more easily influenced by stress than normotensive ones
How does the raas pathway impact trends i essential hyertension. what changes
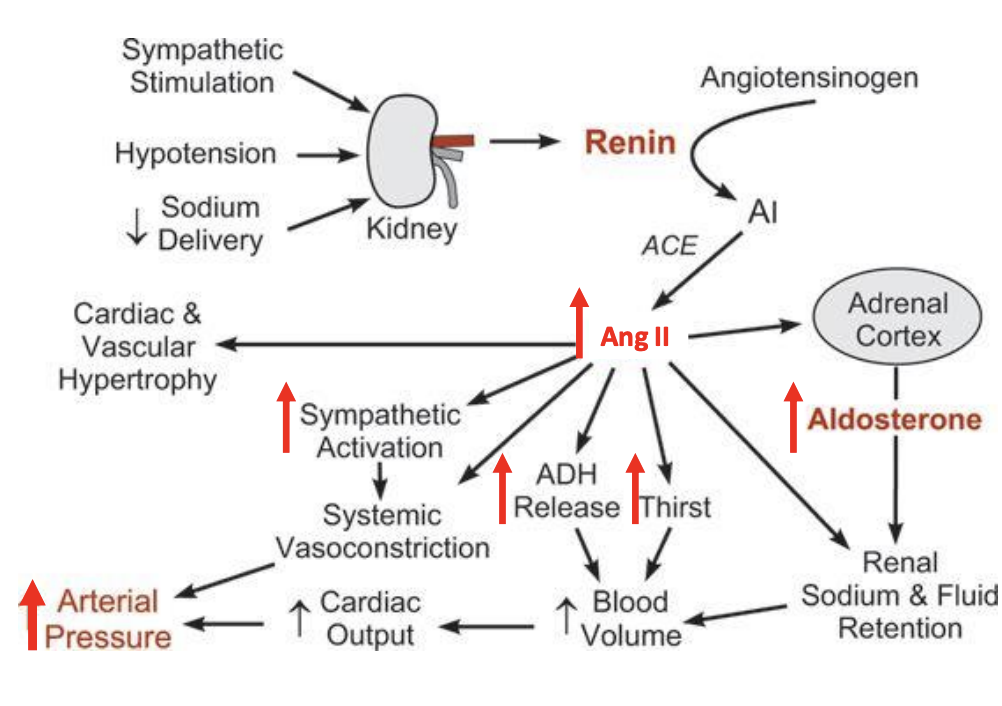
What are te 3 hormonal systems affecting blood pressure and what hormeones are invovled
sympathetic nervous system
norepinephrine
hyperothalimic pituitary axis (HPA)
Cortisol
Rening angiotensin aldosterone system
Angiotensin II and aldosterion
Do general mechanism of hypetension extend to everyone?What does it depend on and what impacts it. how does this impact phenotyping each patient
high strss = higer sympathetic responses
Chronic strss = associated with blutned cortosol/ disruptid HPAaxis activity
symathetic outflow is a diriver of RAAS
sometimes sympathetics are so high that RAAS is supressed
phenotyping each patient wil lbe the best way to manage bp using druigs
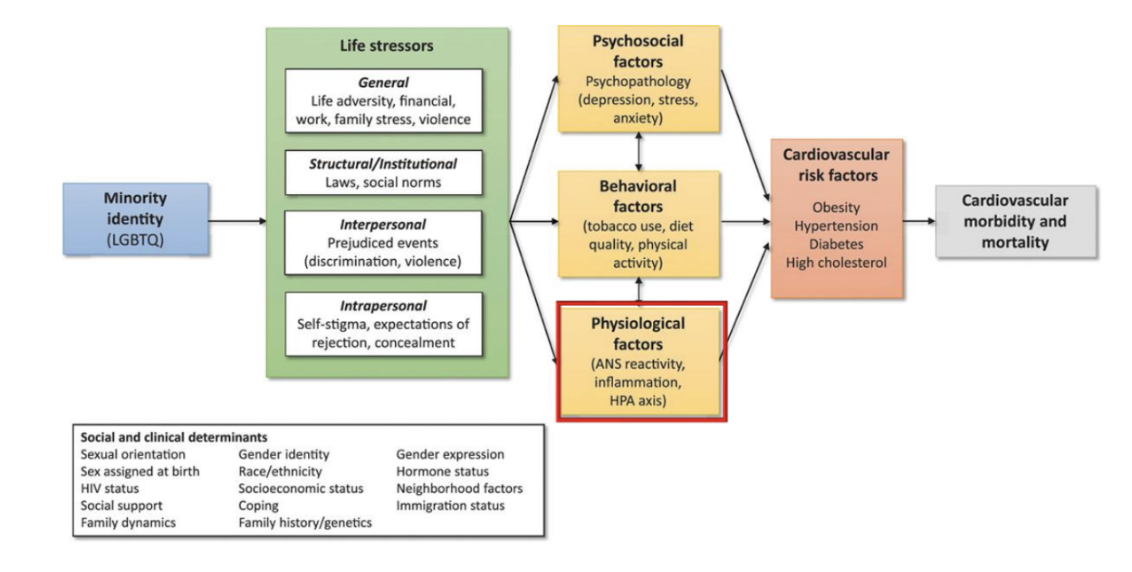
What are some of the statistic s in margionalized communityes. What people are more vulneraeabl e
Race, ethnicity, socioeconomic status, and lgbtq idenity = asoicated with hypertension
Trans men have 4x the risk compared to cis women of MI
Trans women havee 2x compared to cis men of MI
Bisexual women and gay men have 1.2x the risk of having hypertensio
Black adults are 2.2 times more likely to have hypertension vs their white pears
55% of white adults achive bp control while onl y 48% of black and 47% of hispanic and 44% of asian adults did
What are som eof the social determinants of helath
Socioeconomic status
Gender
Sexual orientation
Racialization and ethnicity
What is the minority stress model and draw it out . what are the different layers. life stressors, factors, risk)
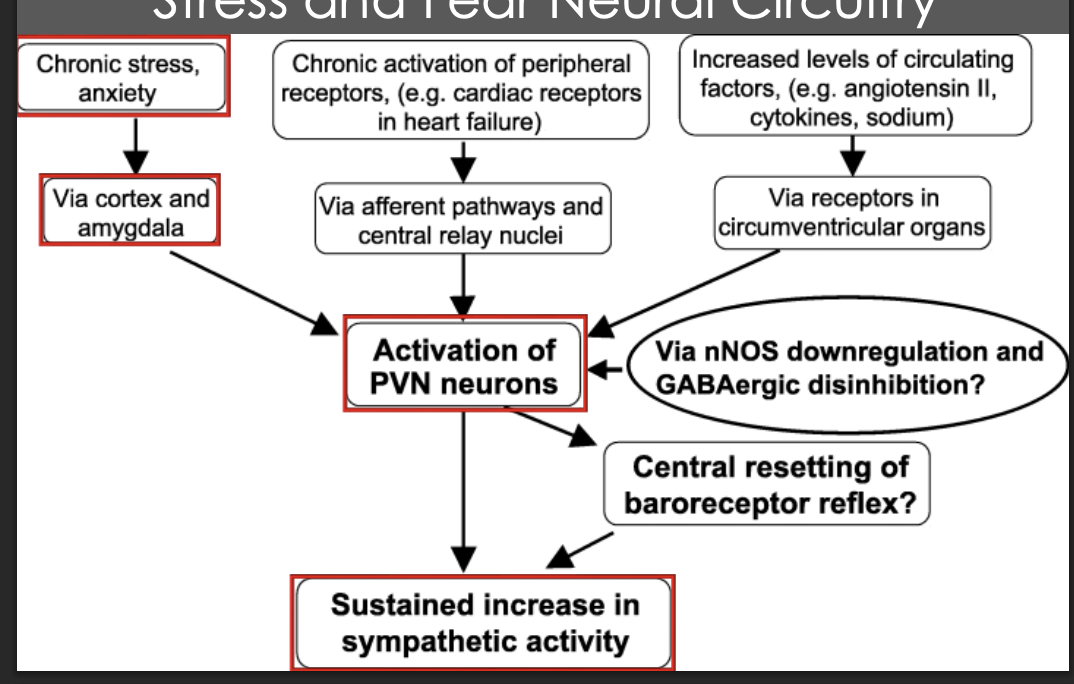
Understand this diagram. stress and fear neaural circutry. what does it mean?
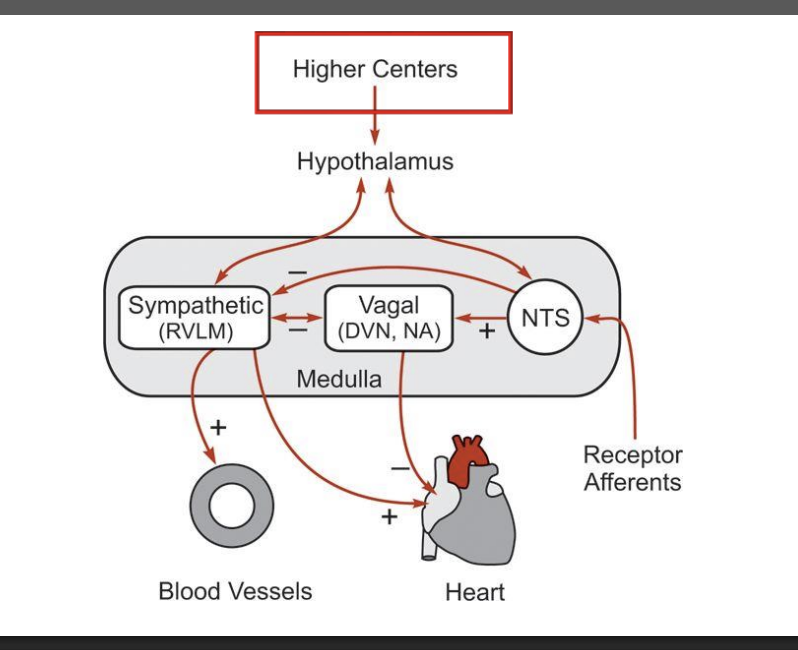
Describe the trends inneural control of sympathetic parasympathetics. higher centers, hypothalamus, nts, vagal, sympathtic, rvlm, dvn, na and the heart and blood vessles
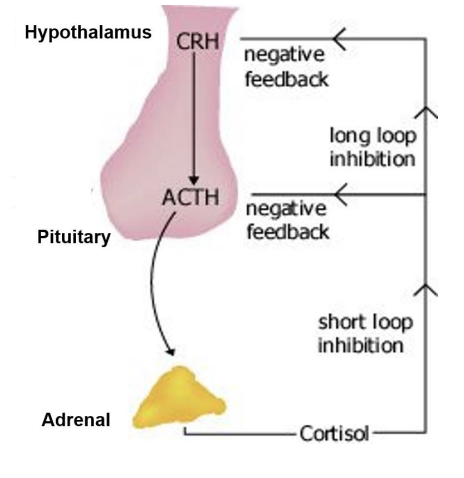
Describe the humoral mechanisms in stress. what is the diagram
There is a chronic stressor
The amygdala signals the hyothalamus
CRH (corticotropic releasing hormone) is released by the hypothalamus
CRH activates ACTH release (adrenocorticotropic hormone) from the ant pituitary
ACTH signals to adrenal cortex to produce and release cortisol
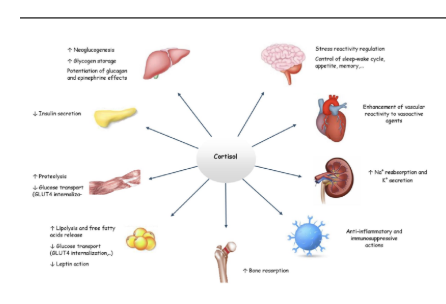
What are some of the organs cortosol affects in the body and how
liver
increased neuogluonsis
inccreased glycogen storange
potenation of glucagone and epinephrine effects
brain
strss reactivity regulation
control of sleep-wake, appetie and memeory
pancreas
decreased inslulin secretion
muscle
reduced glucose transport via glut4 internalization
fat
increased lypolysis and ffa release
reduced glut4 transport
bones
increased bone reabosrption
immune response
antiinfamitory and immunosuppressie actions
heart
incrreased sodium reabsorption and pottasium secretion
kidneys
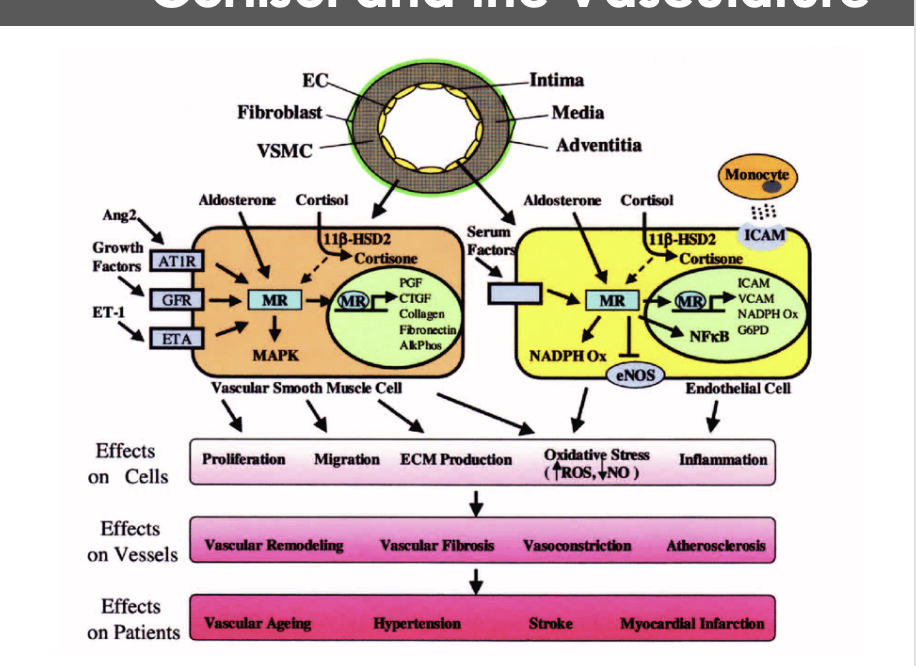
Understanding the diagram: What are some of ehe affects on cortosol in the vasculature- cells, vseesels, and patietns
Cells: proliferation, migration, ecm production, oxidative stress, inflammation
Vessels: vascular remodeling, vascular fibrosis, vasoconstriction, altherosclorosis
Patients : vascular aging, hypertension, stroke, myocardial infarcion
Waht is the effect of chronic stress on MAP
Increased cortisol = increased sympathetic output = increased hr, sv, and arteriolar tone = map increases
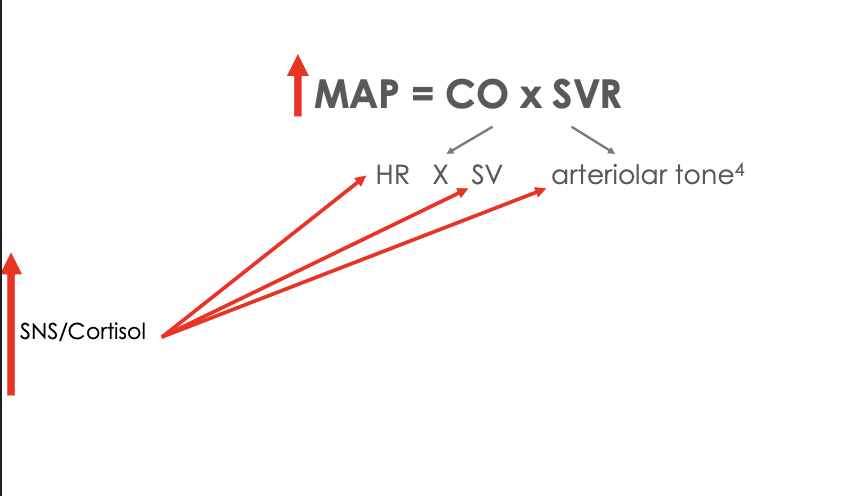
Waht is the effect of elevated BP on the body
Hypertorpy in peripheral arteirols
increased resisntance regardless of SNS input
Cardiac hypertrophy
excsssive fillign pressures
reduced co2
REsetting of the baroreflex
increaed toelrance for higer range of BP
What are some of the interveitions to use in hypertensio n
Map = co x svr
Treating essential hypertension you must adress the main drivers of arterial pressure
Decrease Vasoconstricction
Decrease in vascular sympathetic or RAAS input
Decrease cardiac output
Decrease blood volume and caridac sympathetic input
ACD reginemnd
At1 blockade/ ace inhinhibitors
Calcium channel blocek rs
Diuretics
How effective are treatment methods for hypertension
You can reduce systolic bp to less than 130 and diastolic to less than 80
Decreases stroke risk, decrease chronic kidney disease risk, decreases cardiac death
If left untreated 50% will develo heart faieure
25% develop renal faileure
25% develop cerebral complications
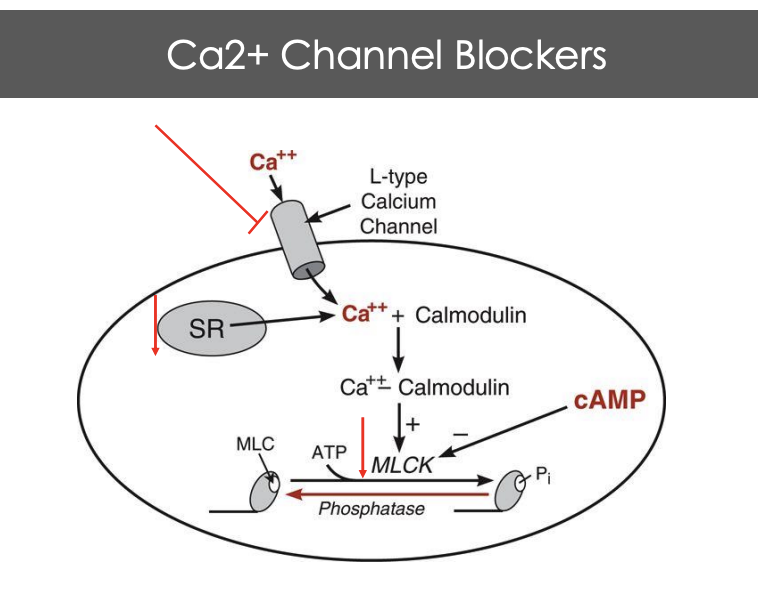
Describe the mechanisms for CA2+ channel blockers
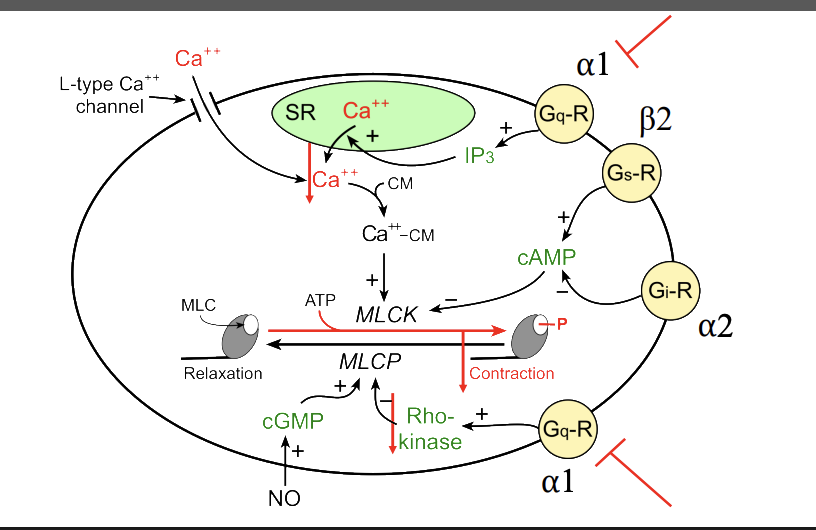
Desccribe the mechanisms for alpha-antagonist on hypertesion (cycole)
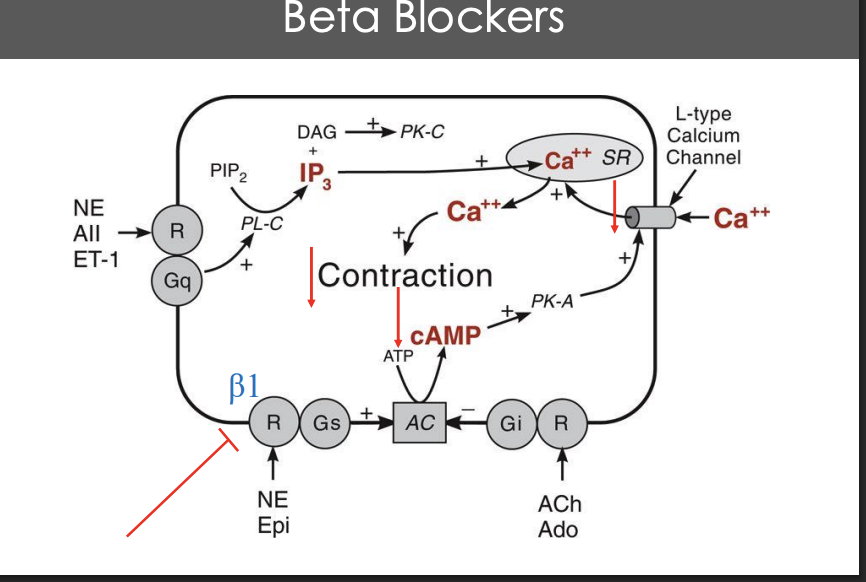
Describe the mechanisms for beta blockers on hypertension (cycle)
What are some of the trends in the data for hypertension in marginalized communities
Lesbian and bisexual women are shown to display more cortisol than heterosexual women
Gay and bisexual mena re shown to display less cortisol then heterosexual men
What are some other ways to manage bp and how
Exercise: mormalizes bp through enhanced parasympathetic tone and baroreflex normality
Access to counseling, cognitive behavioral thray
Effective primar medical care- getting diagnosed without bias and worry
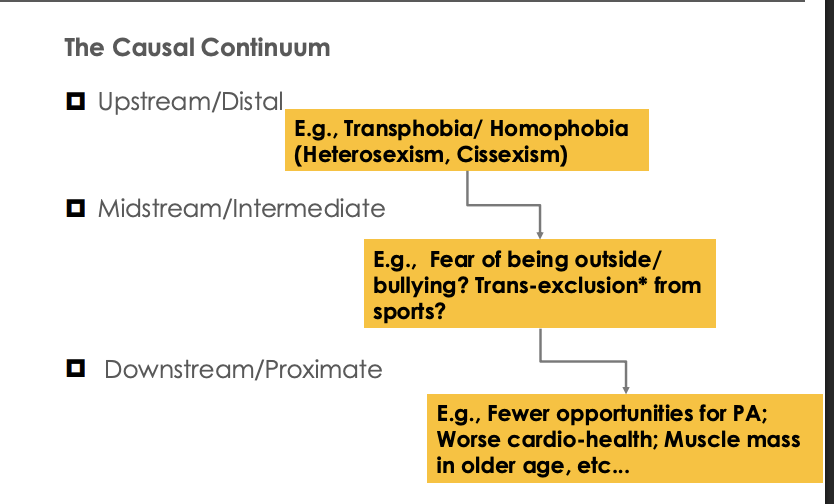
Public health interventions: describe the casual continuum (negative)
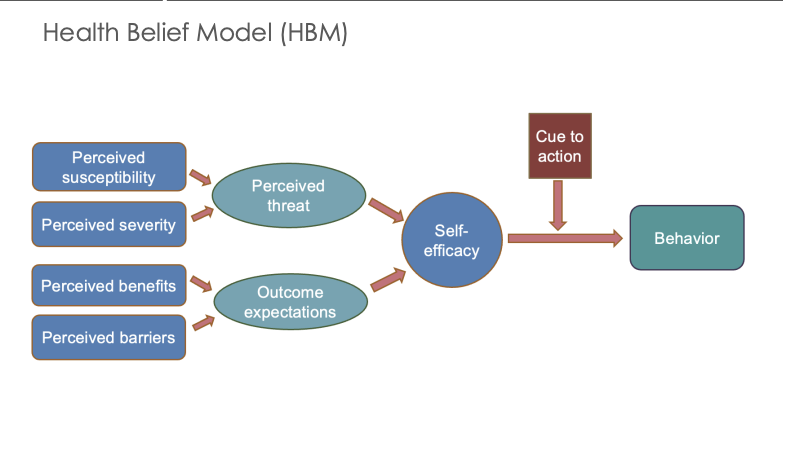
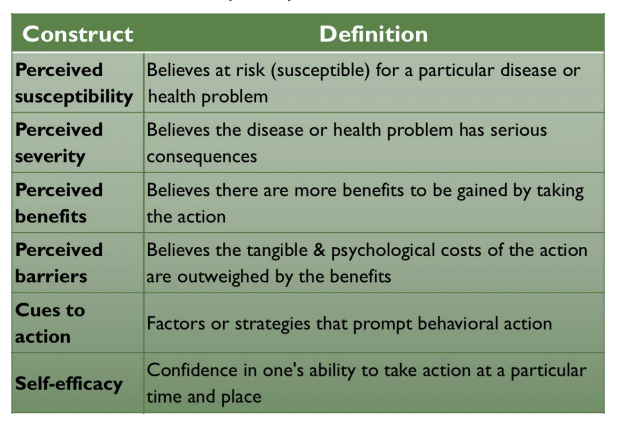
Describe the individual level interveition theory: healht velivf model
How much exercise is resccomended a day for an individual
18-65 eyeasr should participate in moderate activity for atl 30 minutes fo 5 days a week
What are trends in exerfise in the untied states
As of 2020 24.4% of us meet the 2018 acsm a guidelines
Hispanic men and non hispanic black women were at least likely to meet these guidelines
Queer americans reprot higher rates of fear of physical activity due to concerns of discrimination
How can exercise be benificial to ones health and what mechanisms are used
It icreases o2 demand by 15-25 percent
Increase delervery driven by
Incrased co
Redistribution of BF
Inactive organs - working skeletal muscle
Waht are some of the integratvie response of map duing aerobic exercise
Map = co x tpr
Tpr decreaeses during full body aerobic exercises, what sustains the increase in ma
Increases in co (cognitive output)
how do negative feed back loops impact bloodpressure during exercise: what is under the negative feedback loop section
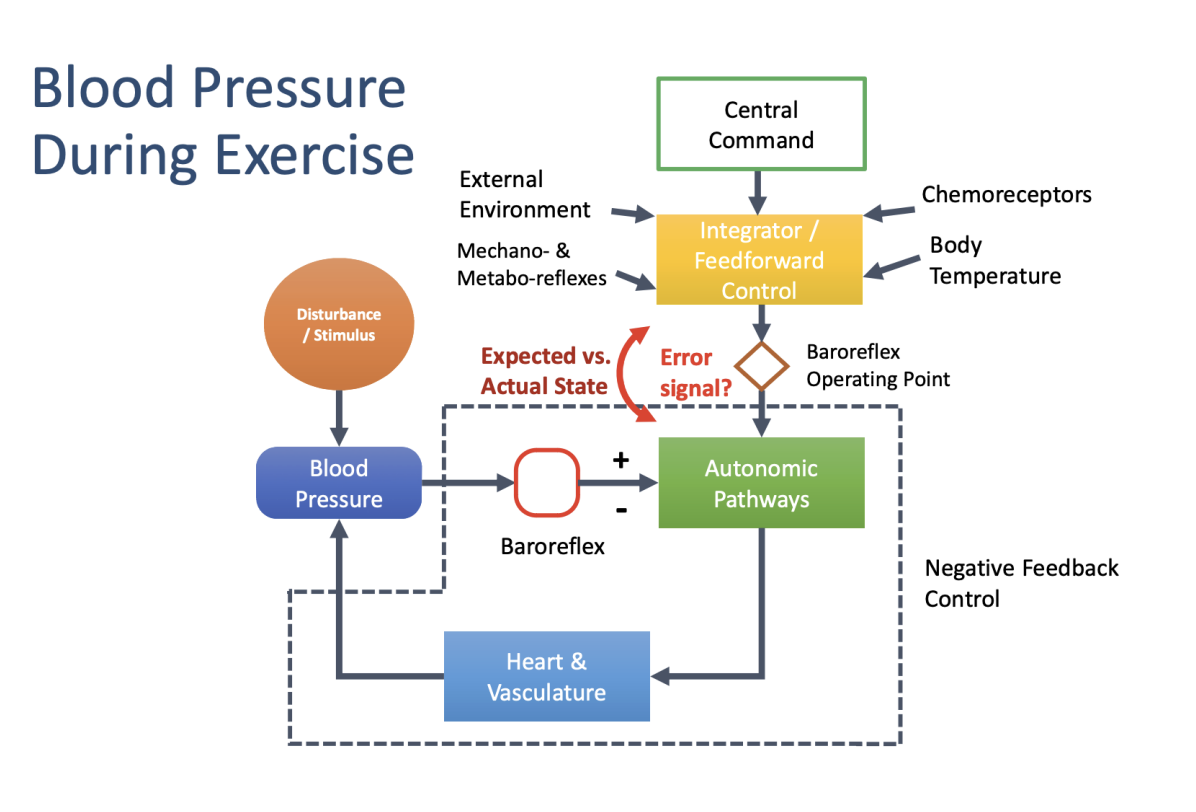
What are the autonmic adaptations of exercises
Altered central (brain) integration/ controller (feedforward and feedback)
Set point and operating point shifts
Decreasing blood pressure and chronic straining
Accuracy of feed forward (expected) controller
Icnreased model accuracy
Improved hr response
Feedforward (Central Command)
When: Before & at onset of exercise
Trigger: Brain → motor cortex
Mechanism: ↑ sympathetic activity, ↓ parasympathetic
Effect on BP: Rapid ↑ systolic BP (via ↑ HR & CO)
Purpose: Anticipates exercise demands
🔄 Feedback (Peripheral Reflexes)
When: During exercise
Trigger: Working muscles & blood vessels
Mechanisms:
Muscle metaboreflex (metabolites: H⁺, CO₂, lactate)
Mechanoreceptors (muscle stretch)
Baroreceptor resetting
Effect on BP: Maintains / fine-tunes BP, prevents hypotension
Purpose: Matches BP to actual exercise intensity
Key idea:
How is bloodflow redistributed during exercise
At rest bloood flow to skeletal muscles is 15-20 percent. During exercise its 80-85% (simulatenous with an increase in sympathetic tone seen during exercises)
What are some of the acute autonomic adaptations to exercise aerobic
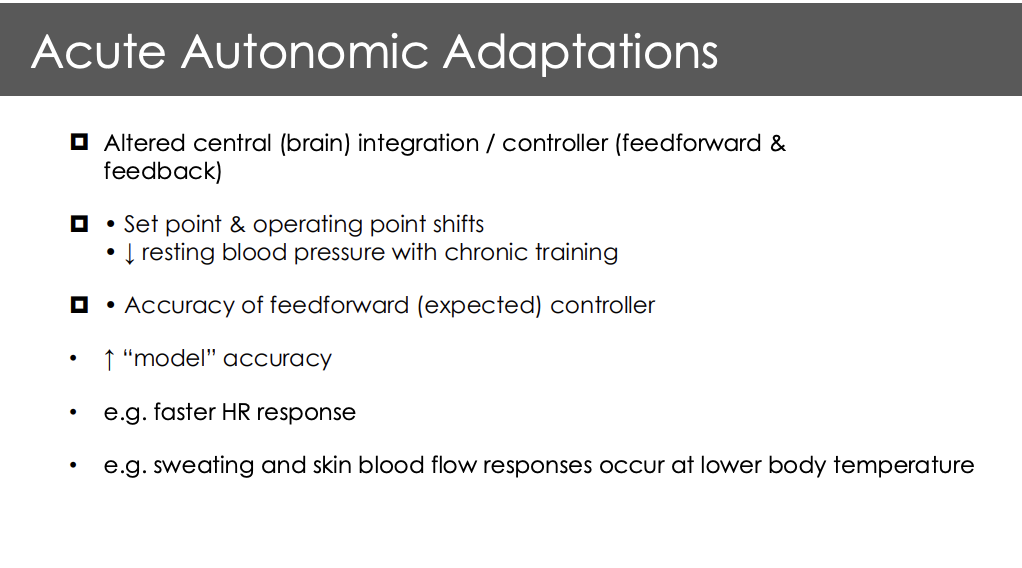
What is and whate drive functional sympaholsis. What are the molecules that allow for vasodialation
Sympathetic transduction is not linear
Signaling molecules such as ATP, ADO, H+, K+, and NO resulting in skeletal muscle vasodielation
Vasoconstrictiong signals from the sns are blunted = vasodialtion of arteriols feeding skeletal muscles (fine tooned) supply = demand
also leads into a long term decrease in systematic blood pressure and diastoli
c pressure
What is msna and What is the effect of MSNA following exercise training (muscle symatheitc nerve activity)
associated with CVD risk and elevated in cv-related disorders
independant predictor of poor prognosis and mortality
if meeting exercise guildlines reduces mortalkit = modulaiton of msna tracks with exercise
wha tis it
A measure of sympathetic nervous system outflow to skeletal muscle blood vessels
What it does:
Causes vasoconstriction in skeletal muscle arterioles
Helps regulate blood pressure and vascular resistance
Effect of Exercise Training on MSNA:
↓ Resting MSNA
↓ Sympathetic vasoconstrictor tone
Improved autonomic balance (↓ sympathetic, ↑ parasympathetic influence)
Mechanisms:
Improved arterial baroreflex sensitivity
Reduced central sympathetic outflow
Improved vascular function and endothelial health
Reduced reliance on the muscle metaboreflex at a given workload
Functional Outcomes:
↓ Resting blood pressure
↓ Peripheral resistance
More efficient BP regulation during exercise
Especially beneficial in hypertension, aging, and heart failure
One-liner to remember:
👉 Exercise training quiets the sympathetic nervous system at rest.
Why do hepef pattens appear to to be
hfpef = the lv of the heart becomes stiff and cant relax properly to fill with blood
it pumps mrore than 50 percent = blood back up
= not enough blood for functional sympatholisis
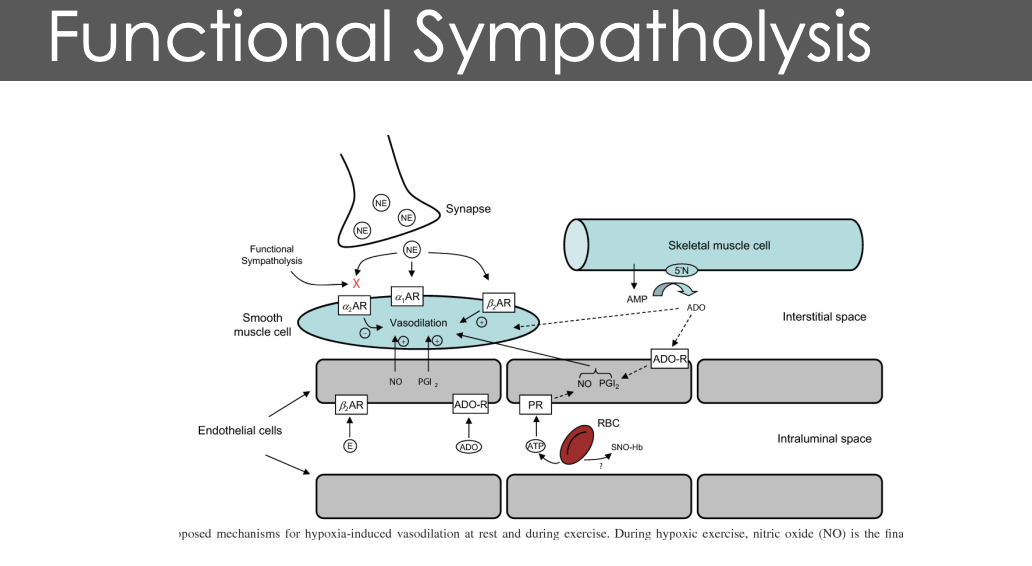
What are some of the changes follwing chronic lv adaptations to long term aerobic exercise
ollowing aerobic exercise, the LF exeprinces greater edv and mass
Increased preload and cardiac load during exercise initiate eccentric hypertrophy
Decreased resting co
Increased working co
At rest and submaximal exercise, HR decreases
Downregulation of beta andregenic receptors
What is the difference between pysiologic and pathologic hypertrophy
Physiological hypertrophy (like an athlete's heart) is a beneficial, adaptive heart enlargement from normal stress (exercise, pregnancy), resulting in normal/enhanced function, normal structure (no fibrosis), and reversible changes. Pathological hypertrophy (from hypertension, disease) is a maladaptive response, causing thickened walls, chamber shrinkage, impaired pumping (leading to heart failure), fibrosis, inflammation, and cell death, often irreversible. The key difference is function: Physiological = enhanced; Pathological = failing
Physiological hypertrophy = exxentric hypertrophy but the chamber space increases in proportion to increased musculature
pathological stimuli
concentri (hfpef)- hpertrophy = reduces lv space
eccentric hypertrophy = prolapse of left ventrical (hfref)
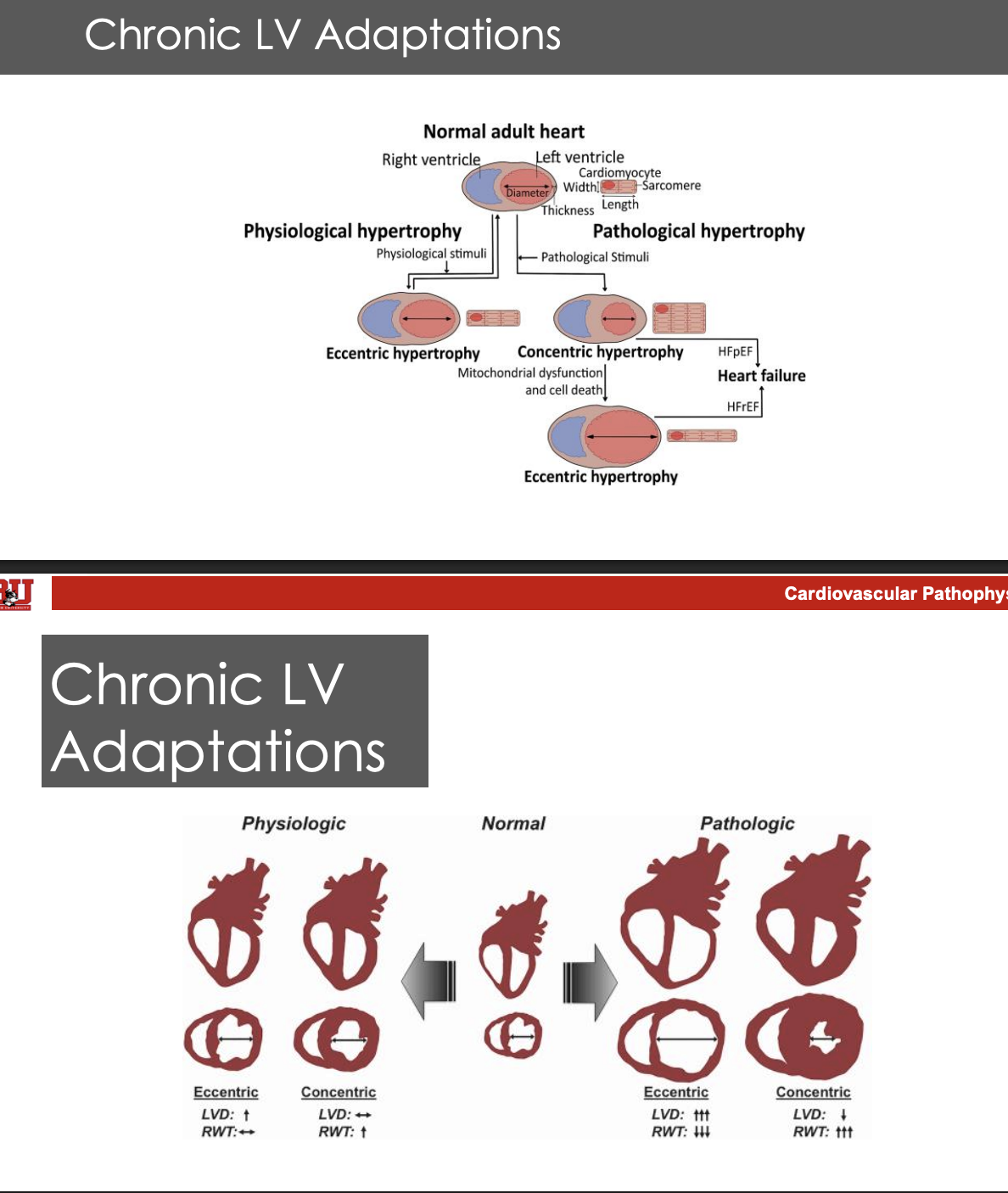
What happens during maximal exercise
the heart’s oxygen consumption increases by 10 fold to increse damand
The athletic heart describes thea daptationi to this increased demand
Cardiac hypertrophy without proliferation of cardiomyocites
Camber size is unaffected
Increased mitochondrial energy production
Pathological hypertrophy is serve remodeling that devolves into hf and dysfunction
What are some of the chronic LV adaptations to aerobic exrecise, venous return to the heart increases? What alters the frank starling mechanism
The frank starling effect
Skeletal muscle pump
Respiratory pump
Venoconstriction
This resuutls in volume overload which increases relative wall thickens (relative to chambers ize)
How does hypertension-induced pathological hypertrophy differ from adaptive (physiological) cardiac hypertrophy?
Hypertension-Induced Pathological Hypertrophy
Cause: Chronic ↑ afterload (long-standing HTN)
Duration: Chronic increase in cardiac demand
Geometry: Concentric hypertrophy → may progress to dilated cardiomyopathy
Cellular changes:
Myocyte thickening with fibrosis
Altered gene expression
Reversibility: Not fully reversible
Outcome: Impaired relaxation, ↓ compliance, risk of HF
Adaptive (Physiological) Hypertrophy
Cause: Temporary or intermittent ↑ cardiac demand (e.g., exercise)
Duration: Short-term / training-related
Geometry: Proportional chamber & wall growth
Cellular changes:
Minimal/no fibrosis
Normal cellular architecture
Reversibility: Fully reversible
Outcome: Maintained or improved cardiac function
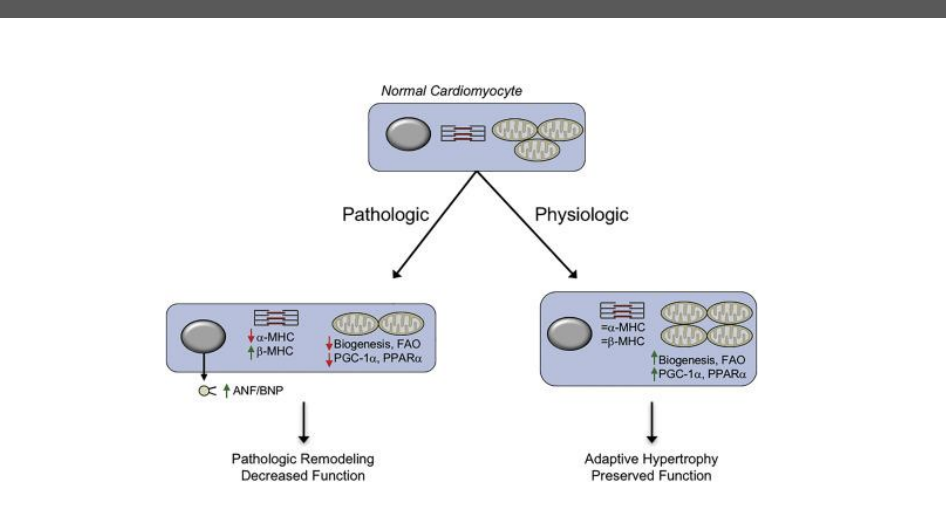
How do chronic pathological vs physiological cardiac hypertrophy differ at the gene-regulation level?
Pathological Hypertrophy (e.g., HTN, Aortic Banding)
Trigger: Chronic pressure overload
Gene regulation: Reactivation of fetal gene program
↑ ANP (atrial natriuretic peptide)
↑ BNP (B-type natriuretic peptide)
↑ β-myosin heavy chain (β-MHC) (fetal isoform)
Metabolism:
↓ fatty acid oxidation (FAO)
↑ glucose metabolism
Cellular outcome:
Fibrosis
Reduced efficiency and contractile dysfunction
Key evidence:
Aortic banding induces this fetal gene activation
Physiological Hypertrophy (Exercise Training)
Trigger: Intermittent, adaptive ↑ cardiac demand
Gene regulation: No fetal gene reactivation
ANP/BNP not pathologically elevated
No ↑ β-MHC
Metabolism:
↑ fatty acid oxidation (FAO)
Cellular outcome:
Normal structure
Preserved or enhanced function
Key evidence:
Swimming alone or swimming + aortic banding prevents fetal gene activation
Bottom line:
👉 Pathological hypertrophy reverts to a fetal gene + glucose-dependent state
👉 Physiological hypertrophy maintains adult gene expression + FAO dominance
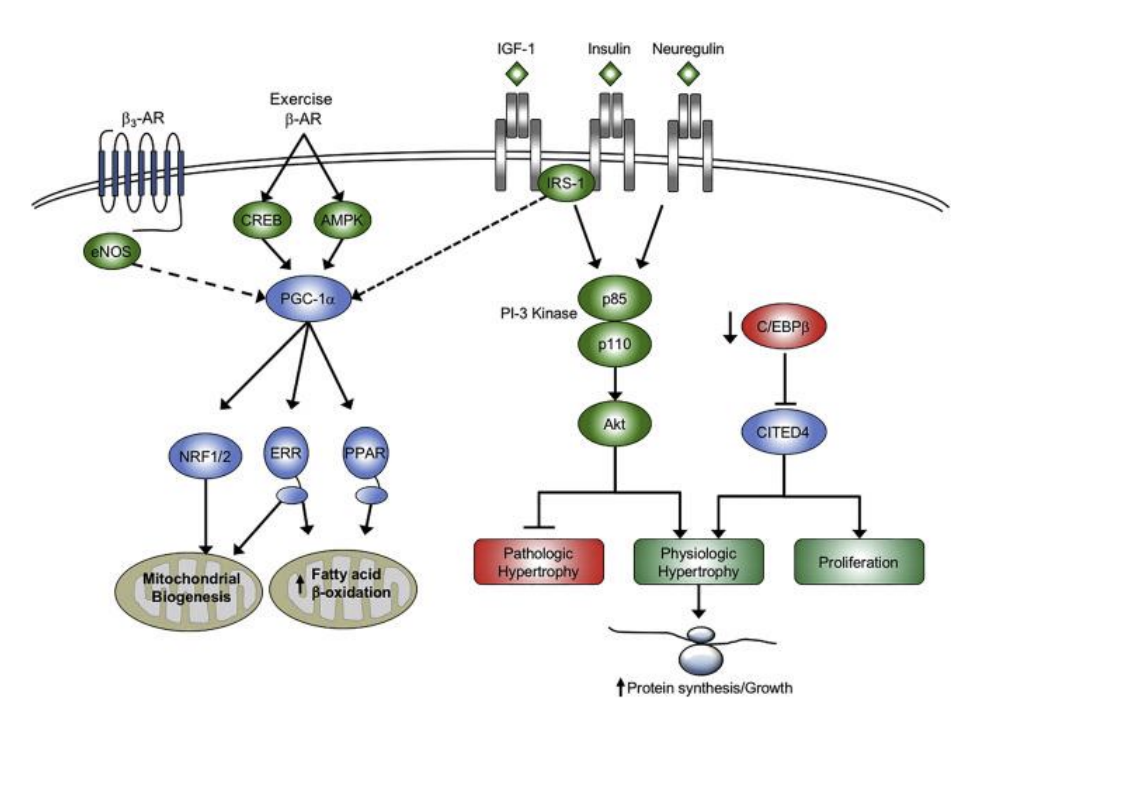
What are some chronic mitochondiral adaptations to exercise
Mitochondrial biogenic adaptation following exercises decreased in disease sates
Likely follow similar adatations as seen in skeletal muscle
Upregulation of citrate synthase
Increased cardolin levels
Etc complex activity (limited by nDNA and mtDNA)
mtDNA copy number (respond to deconditioning)
Keay regulator in cardiac and SKMSC - peroxisome-activated receptors gama coactivator 1 alpha (pgc1-alha)
Try to understand knock dowon models
What are teh long term adaptations to exercise
Aerobic training: increased heart diameter
Increased flow mediated dilation
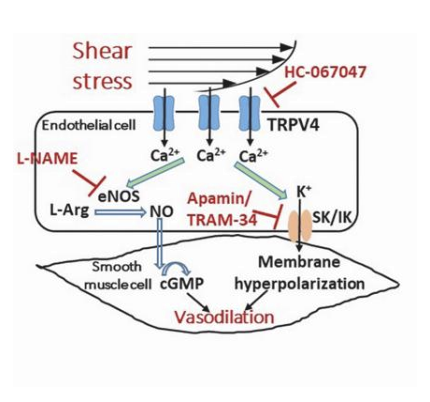
Improves sympatholica bility
Co increases = bloodflow increases = redistrbutes to skeletal muscles = increased shearing forces = enos activation = enhanced no production = begf = arterioal growth = enlargement
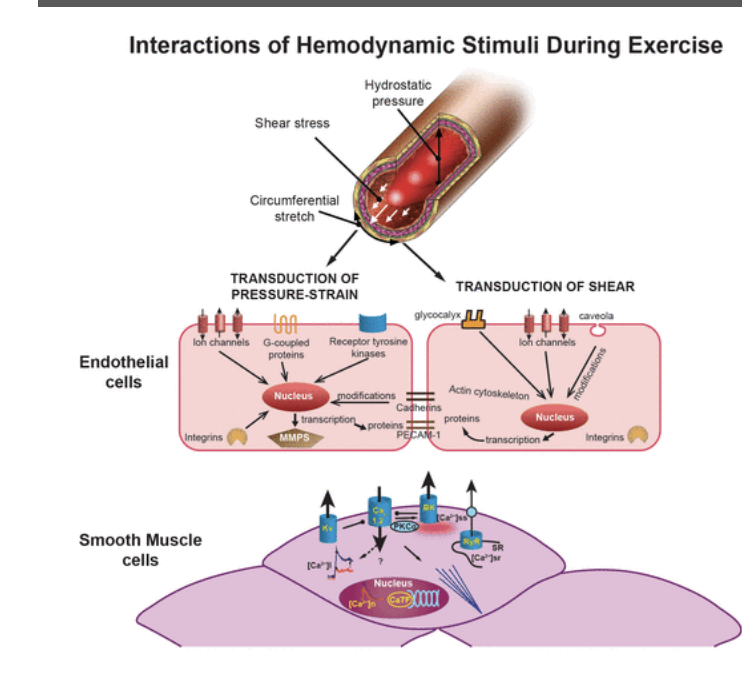
What are some of the interactions of hemodynamic stimuli during exercise
Increased flow induce EC proliferation
Increased pp and distensitibilty arteries result in cynical cercumfrential strain
Decreased intracellular ca2+ despite increased influx
Increased slow release from the sr
Exercise activates kv channels
Ec reorganize perpendicular to force vectors = matrix and gf modulation
What are some of the chronic vascular adaptations
Increased hr and sbp iniduce circumfrential strain on the aorta parealleed with peak flow
Strain activates endothelium independent vasodiletory pathways
Enos
Edhf
Chronically elevated BP: ROS+ adheaasion molecules >> enos
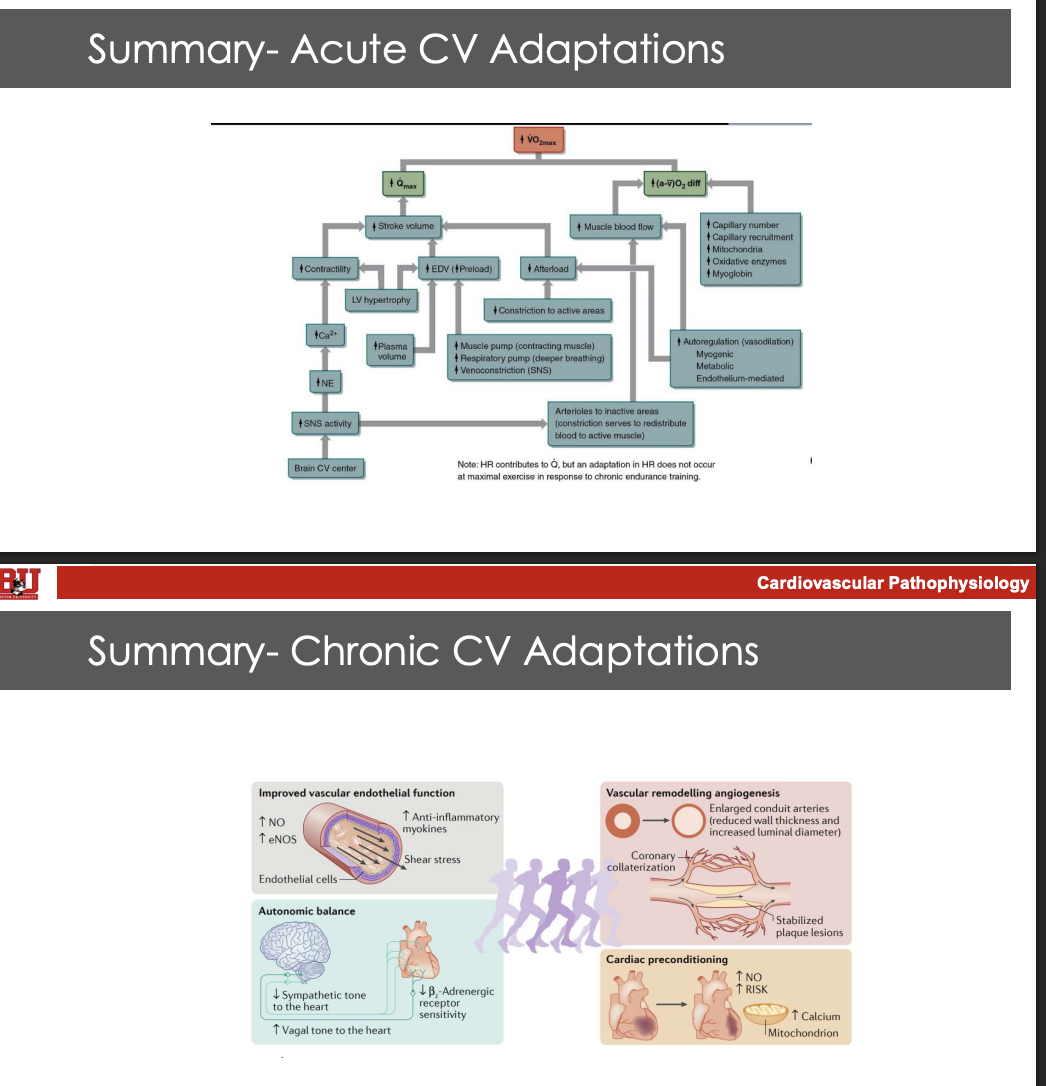
What are the general chornic CV adapatatoins fo rthe following locations: endothelial function, autonomic balance, vascular remodeling, and cardiac preconditioning
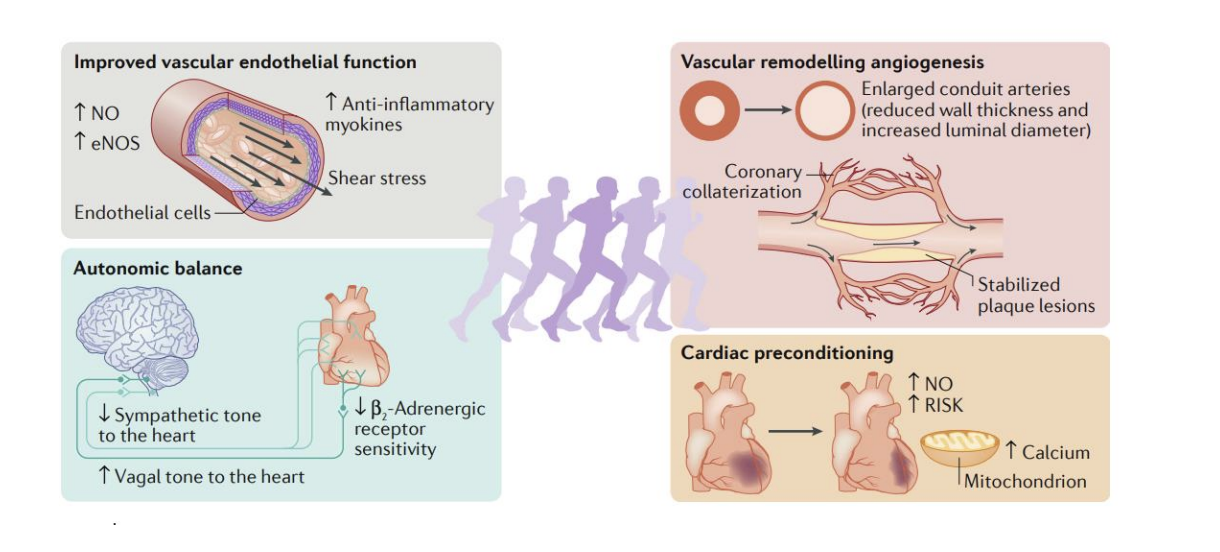
How many people ar eliing with heart faleure in the us today
Over 6million with a 46% increase
What are the symptoms of heart failerue
Heart is unable to supply adequate blood flow to peripheral tissues/ organs or is unable to do so only at elevated fillign pressures
Most commonly includes the left ventricle
Mild heart failure initially resents as reduced exercise capacity and the development of shortness of breath (exteritional dyspnea
Sever forms patients present dysnpea at rest
Patitents will likey have a significant pulmoary or systemic edima ina more sever form
What are the intrinsic causes of heart faileure: note there ar 6
Intrinsic causes
Coronary artery disease (CAD) * is the most common
Reduces blood flow/O2 to the heart
Myocardial infarction *very common
Incrased demands lead to functional changes
Valvular disease and congetial defects
Cardiomyothopies (intrinsic disease of the myocardium)
Bacterial, viral, alcohol induced unknon origin (idiopathic)
Intrinsic causes continued
Ineffecve or noniffective myocardis
Inflammation of the myocardium
Chronic arrhythmias
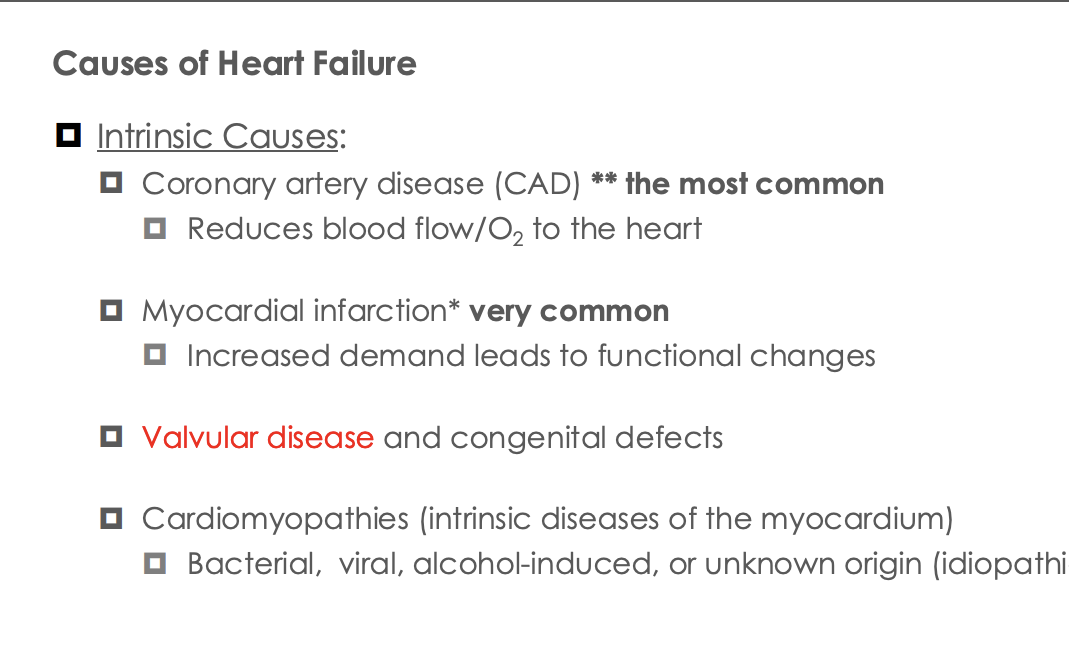
What are teh different types of cariomyoatheis
Dialated cardiomyopath
Results in valvar dilation
Hypertrophic cardiomyophphy
Reductuction in in chamber space in the left ventricle
What are the extrinsic causes of heart failure (hit there are 4)
Sustained elevations in afterload (uncontroled in hypertension)
Increased stroke volume
Volume load (too much pree load) = arterioul venous shunts
Increased body demands
High output failure
Thyrotoxiosis - an excess of thyroid hormone in the body
Pregnancy ‘
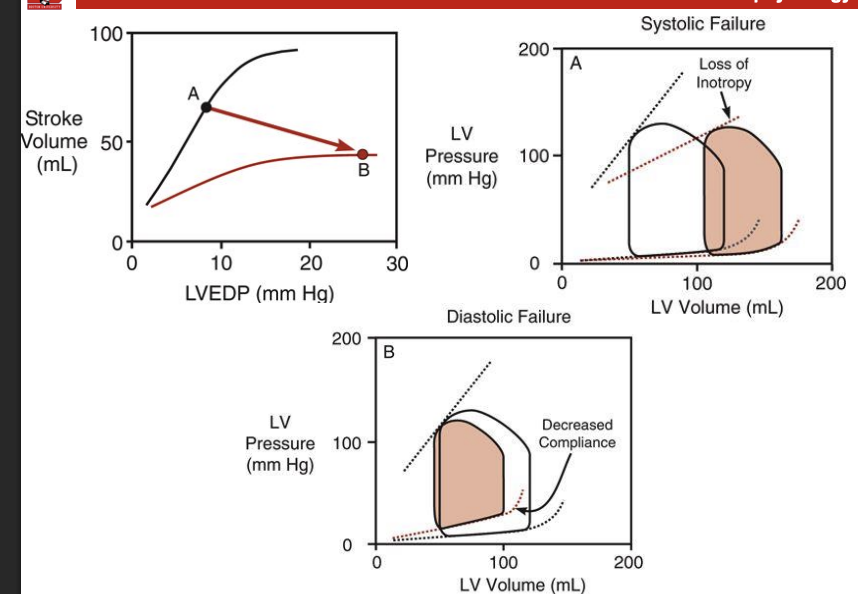
What is the differene between HFpEF and HFrEF
HFrEF (when the heart) (systolic faliure)
s main pumping chanmber. becomes weak and cant squeese hard enough to sufficently pump sufficient blood tho the body (less than 40%)
Result from impaired ability of the heart muscle to contract = reduced sv and co
Caused by changes in cellular signal transduction mechanism s and excitation-contraction coupling that impair ionotrypy
Causes a downward shift in frank starling curve
HFpef (diastolic falirue) (heart failure with preserve ejection fraciton, condition where the heart bcomes too stiff and cant ill properly but ejection fraction is still mroe than 50%
casuses reudced sv and co
Caused by either:
Decreased ventricular compliance
Most commonly ventricular hypertrophy
Impaired relaxation (decreased lusitropy)
Other causes:
Hypertrophic cardiomyopathy
Normal age-related changes to cardiac structure
Type 2 Diabetes
What is the effect of hfref on the frank straling curve and preload
increase in preload to make way for the lack in blood = activates frank starlign to make up for loss of ionotorphy
no compensary increase = decline in sv would be creater for a loss of ionotrophy
the heart will not be able to pump efficenlty and be exhusted if this persist since the sarcomeres will be pulled to its max leigth
the lv dialates = more sarcomeres in serices = more compliace = more filling without increases in pressu re
draw the frank starling curve for hfref
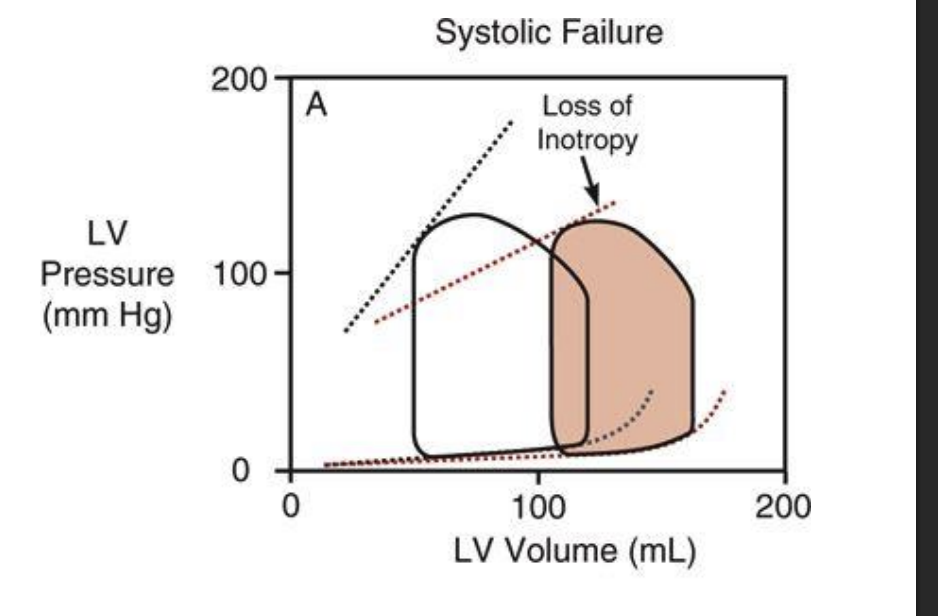
Draw out the frnak starling curve for HFpef. how does that impact sv
dpending upon the relative change in sv and edsv the ejection fraction might not chnange = ejection fraction is only useful as an indicator of systolic falieure
elevated diastolic pressures = pulminary edima (increased afterload)
peerhiperal edima and abodminal sceites with r ventricular flaure
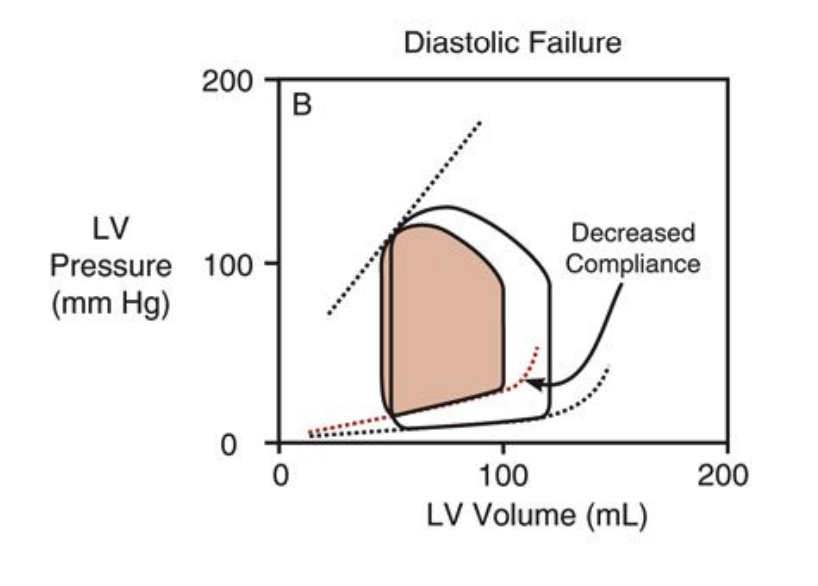
Draw out the frank starling curve for both systolic and diastolic failure
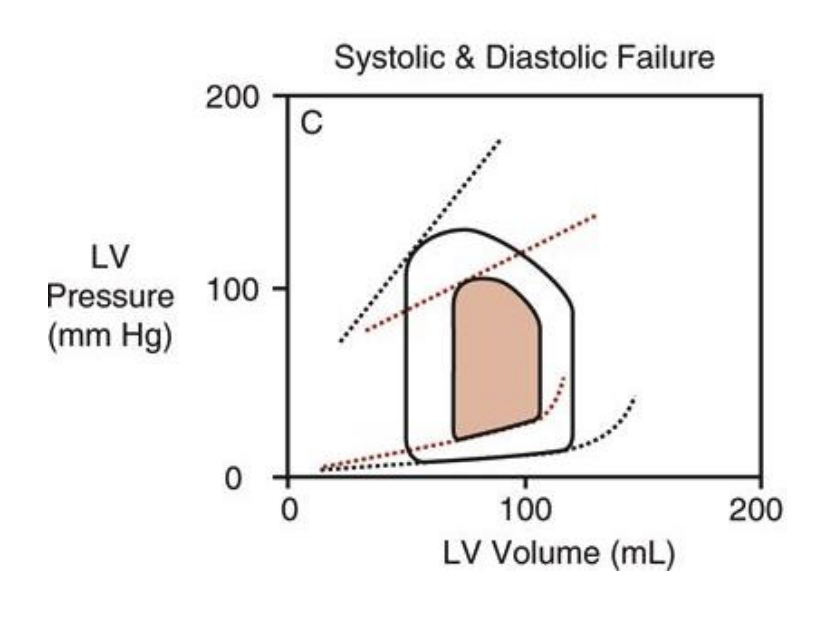
What is congenstion and what are the signs of congesion (hint there are 5)
definitions: chronic condition where the heart muscle weakens and can't pump enough blood to meet the body's needs, causing blood and fluid to back up (congestion) in the lungs
signs:
Dyspnea (exertional → resting):
Shortness of breath caused by fluid congestion in the lungs; it first appears during physical activity and, as congestion worsens, occurs even at rest.Orthopnea:
Shortness of breath when lying flat due to increased venous return and pulmonary congestion; relieved by sitting up or using multiple pillows.Cough:
Often a dry or frothy cough caused by pulmonary congestion and fluid accumulation irritating the airways.Peripheral edema (pitting edema):
Swelling of the lower extremities due to venous congestion and fluid retention; pressing on the swollen area leaves a temporary indentation.Paroxysmal nocturnal dyspnea (PND):
Sudden episodes of severe shortness of breath at night caused by redistribution of fluid to the lungs during sleep, forcing the person to wake up gasping for air.
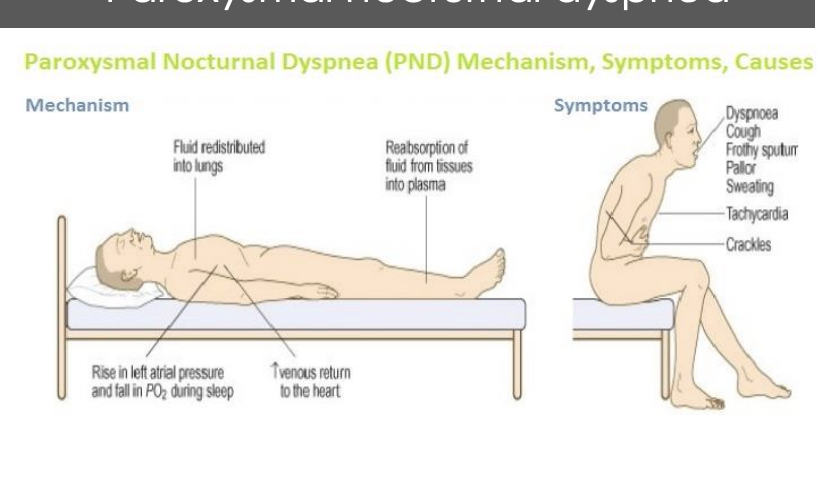
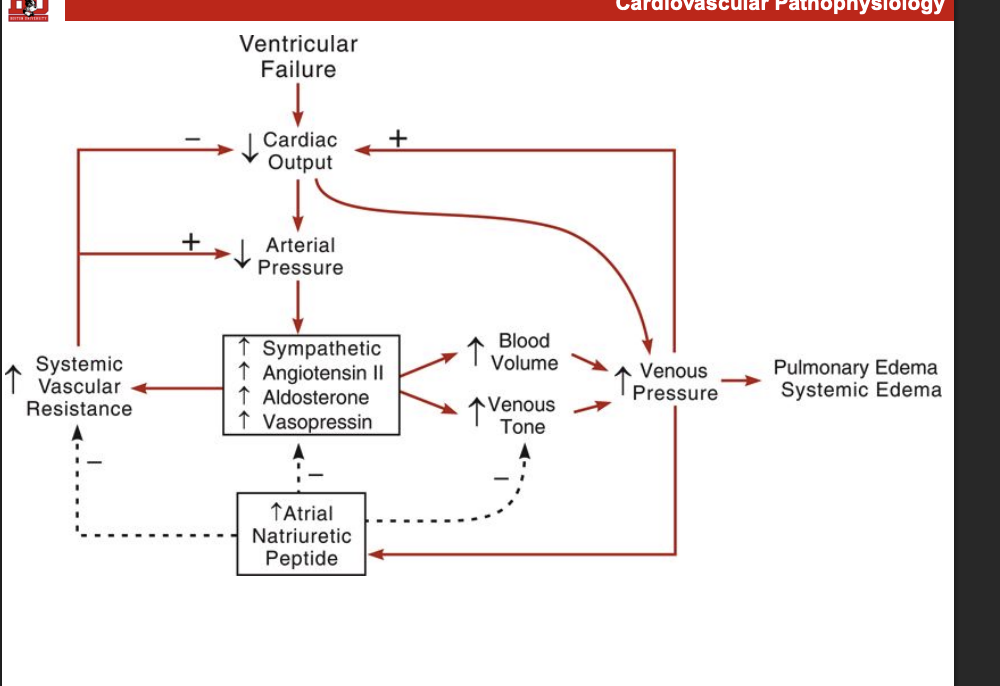
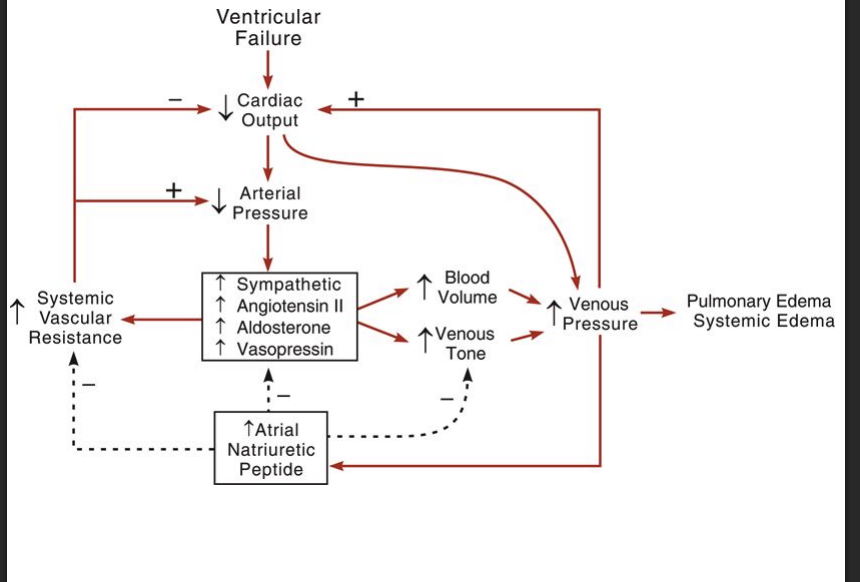
What are some of the comensary mechnanisms in heart faileure
Goal: icnreas MAP
symathetic activity is increased
barroreflex reseting
increased fluid retention
increased RAAs activation
increaed ADH release
increaed catechlamine release
arterial and venous vaso constriction
What are ways to treat hf and what are the appraches and goals 3 and 4
3 primary goals of treatment:
1. Treat symptoms of edema & dyspnea
2. Improve CV function & exercise capacity
3. Reduce mortality
4 approaches to treatment
1. Reduce venous pressure – reduce blood volume & vasodilate
2. Reduce afterload - vasodilate
3. Increase ventricular inotropy – inotropic agents
4. Use beta-blockers…? – reduce mortality
Unsure of the mechanisms of how this is effective
5. Angiotensin receptor-neprilysin inhibitor (ARNI)
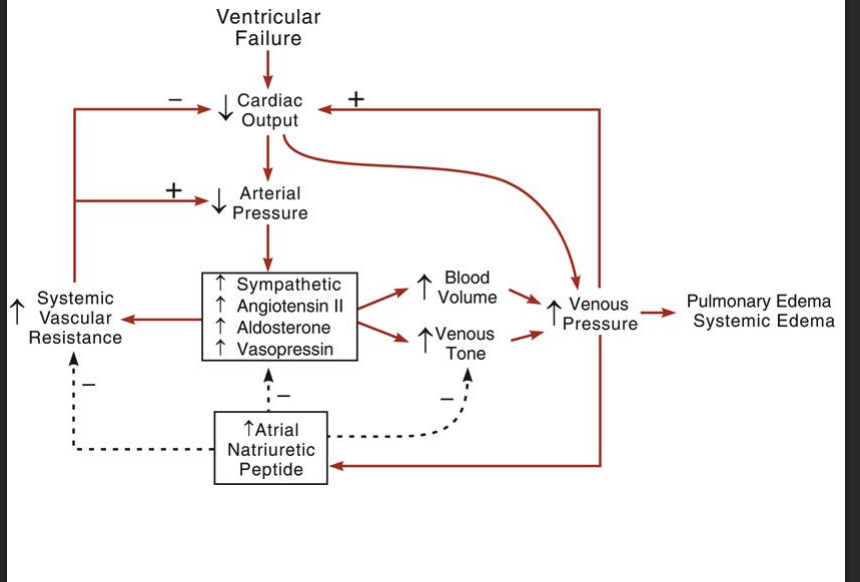
Waht are sglt2 inhibitors
What they are:
Medications originally developed for diabetes that block the sodium–glucose cotransporter-2 (SGLT2) in the kidneys, increasing glucose and sodium excretion in the urine.Use in HF:
Reduce hospitalization and mortality in heart failure (both HFrEF and HFpEF), even in patients without diabetes, by decreasing fluid congestion, lowering preload/afterload, and improving cardiac and renal outcomes.
Examples include dapagliflozin and empagliflozin.
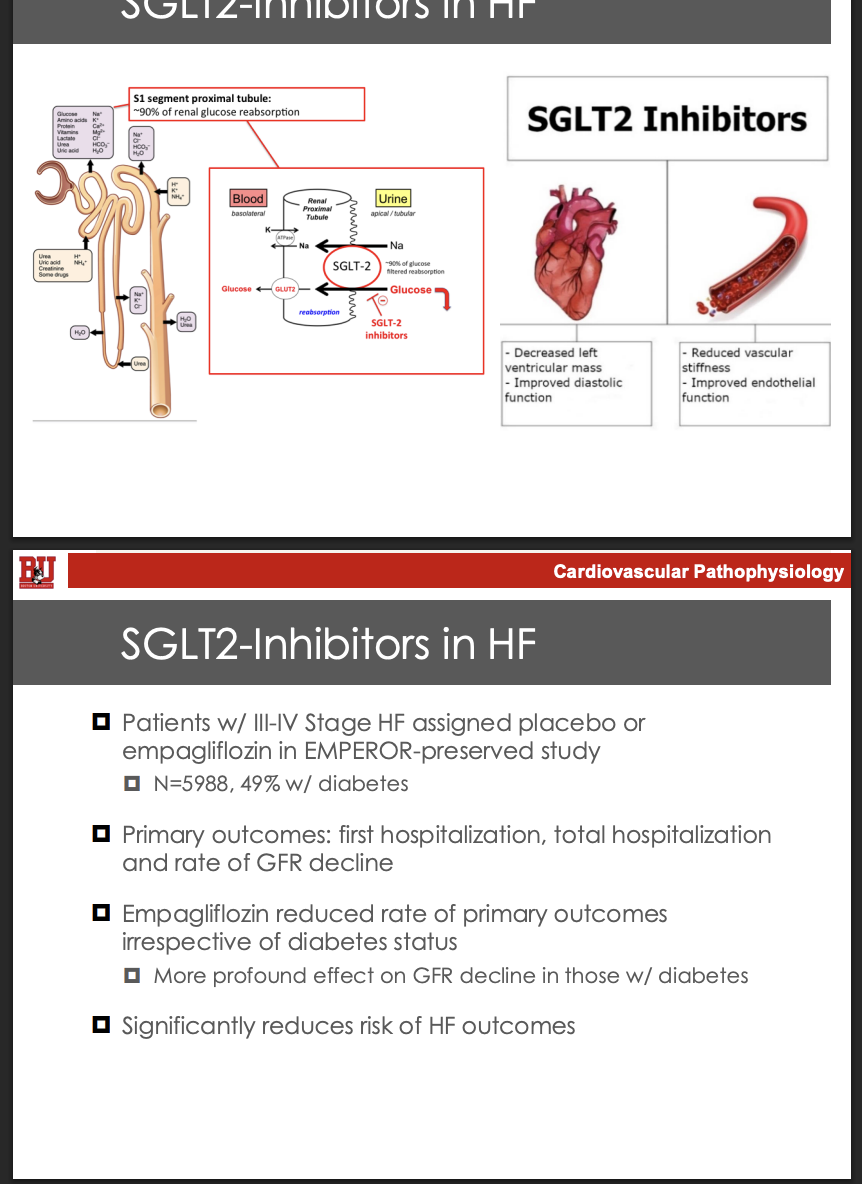
What are some of the fucntions of mitochondria and what are functional,intermediate, and dysfuctional
Functional
Atp production
Growth and adaptation: biosynthesis, protein modification, mitochondrial nuclear communication
thermogenisis
Intermediate
Ca2+ transport: metabolic stimulation, stress response, ca2+ homeostasis
ROS: oxidative stress, redox regulation, cell signaling
Dysfunctional
Inflammation: mtDNA or peptides ROS
Cell death: mptp opening, cytochrome c relase, Energy deprivation
What are the fule and give ofs of mitchondria
Calories (fuel)
Gives off: thermal energy, molecular energ (atp) oxidants
The ehart consumes ~ 5600 l of oxygen and 6k of atp daily
What are th 13 oxidative phosphrilation subunits
7 comple 1
1 complex 3
3 complex 4
2 complex 5
22 translate
2 rrnas
how many copies of the mitochondrial DNA are in a single mitochondrion
a. 5 -10 copies
What is mitochondrial heteroplamsy
When a single cell contains a mixture of different mitochondrial DNA types usually in combinations with wild types
60 % of individuals in the population carry
How are mitchondrial heteroplasmic varients inherited
Maternally (they are accuired) (male variants are rejected)
What genes encode oxidative hsphorilation
Nuclear and mitchondrial genes
do mitocondrial genetic varients play a role in cardiovascular idsese: what are some of the genetics of mitochondrial disae
281 different mitochondrial games either nuclear are imlicated
47% are varients of unknown significance
Heterognety within familes carryign the same pathologic variant
33% of neonatal/pediactric disease onset
75% of adult disesse onset
Accumulation of pathologic mitochondrial DNA varient
Mitochondrial heteroplasmy → energetic failure + oxidative stress
Heart is especially vulnerable due to high ATP demand
Disease appears once mutant mtDNA crosses a functional threshold
Drives cardiomyopathy, heart failure, and progression with aging
One-liner to remember:
👉 Mitochondrial heteroplasmy compromises cardiac energy supply, pushing the heart toward failure when mutant mtDNA reaches a critical level.
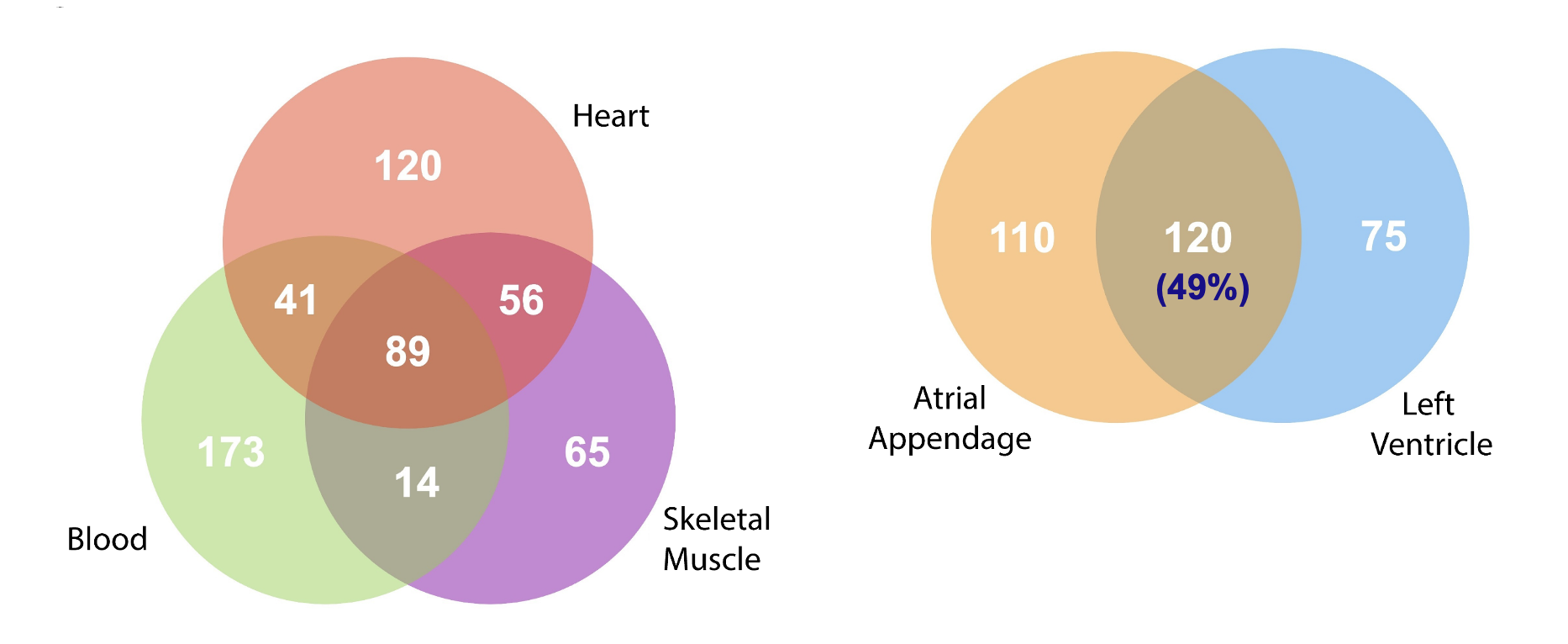
Define mitochondrial heteroplasmy and its relationship to cardiovascular disease and aging.
Mitochondrial Heteroplasmy is the state where a single cell contains a mixture of different mitochondrial DNA (mtDNA) types, including pathogenic variants alongside wild-type mtDNA
The number of heteroplasmic variants increases with age in the heart (atrial appendage and left ventricle), suggesting implications for age-related CVD
They have a higher risk than people who formerly smoked or never smoked at all age groups
Do mitochondrial genetic variants in teh general populatiosn associate with cardiovascular disease
Mitochondrio haplogroups associate with cardiovascular diseases. Mitochondrial heteroplasmy → energetic failure + oxidative stress
Heart is especially vulnerable due to high ATP demand
Disease appears once mutant mtDNA crosses a functional threshold
Drives cardiomyopathy, heart failure, and progression with aging
One-liner to remember:
👉 Mitochondrial heteroplasmy compromises cardiac energy supply, pushing the heart toward failure when mutant mtDNA reaches a critical level.
What are the specific
how are pluirpotent cells introduced
They do not reuqire the use of embryos
They are derived from donated skin/ blood ccells from a patient
Indefinelty self renewing
Ability to become any cell type
Patients own genetic background
How does mitochondrial genetic variation in blood relate to that in the heart
Only 13% of heteroplasmic varients were shared between all tissues
50% were shared in the left ventricle and atrial appendage
As you age there are more variants in the atrial appendage, left ventricle but less in the blood
Are mitocondrial disease associated with cardiomyopathy
yes
what hapens when heteroplasmic varients accumulate in the body
they are assoicated with mitochondrial disease onset
What is the difference between heteroplasmic varients in the heart compared to the blood
Only 13% of heteroplasmic varients were shared between all tissues
50% were shared in the left ventricle and atrial appendage
As you age there are more variants in the atrial appendage, left ventricle but less in the blood
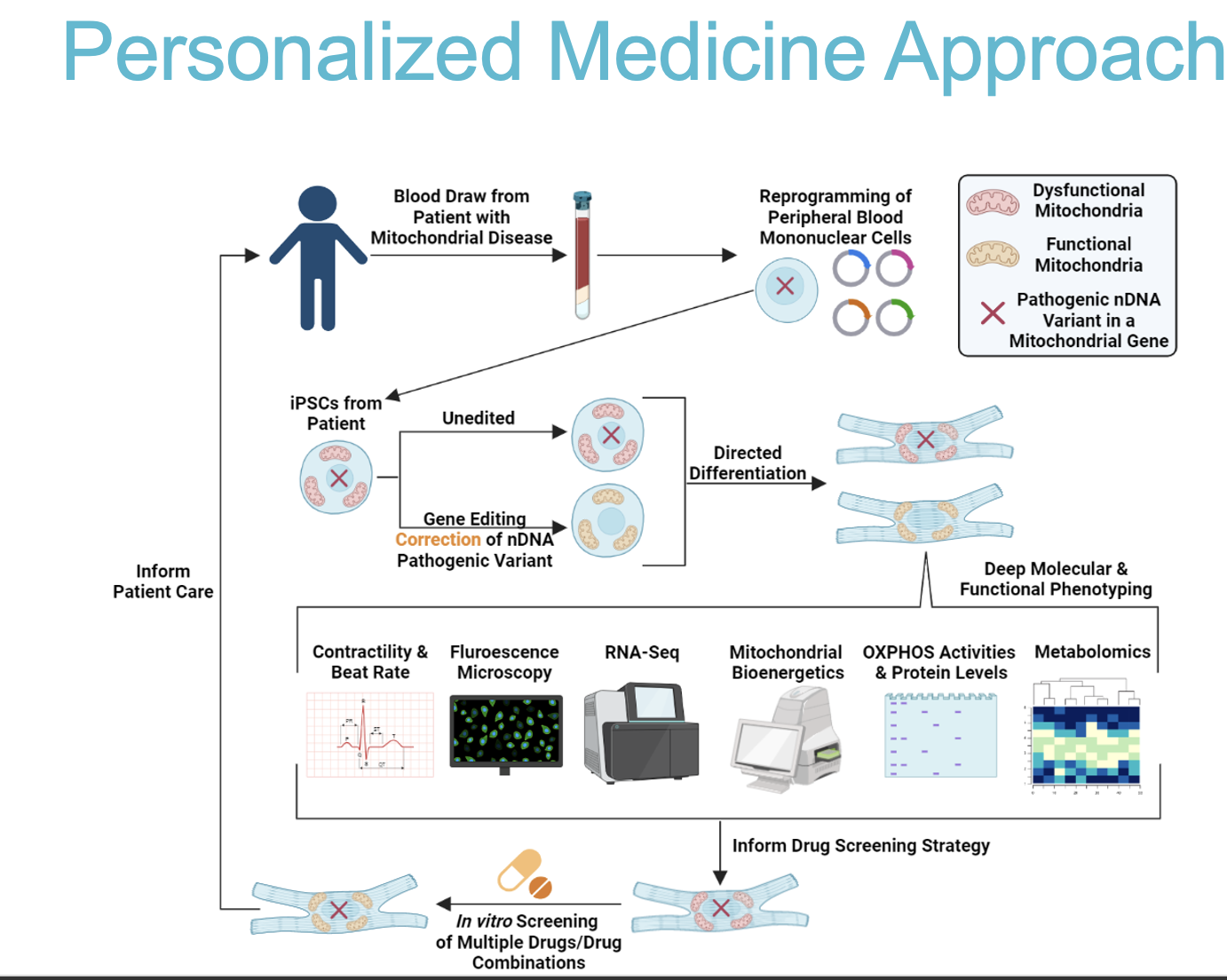
Describe the personalized medicine approach for studying mitochondrial genetic variants using iPSCs.
Reprogramming: Peripheral blood cells from a patient are reprogrammed into Induced Pluripotent Stem Cells (iPSCs). 2. Differentiation/Editing: The iPSCs (representing the patient's genetic background) are directed to differentiate into cardiomyocytes. Gene editing techniques may be applied to correct pathogenic nuclear DNA variants. 3. Phenotyping: These cells undergo deep molecular and functional phenotyping (e.g., contractility, OXPHOS activity) and are used for in vitro screening of drugs/drug combinations.
What is the influence of mitchondiral heteroplasmy in mitochondiral diseases and onset and aging

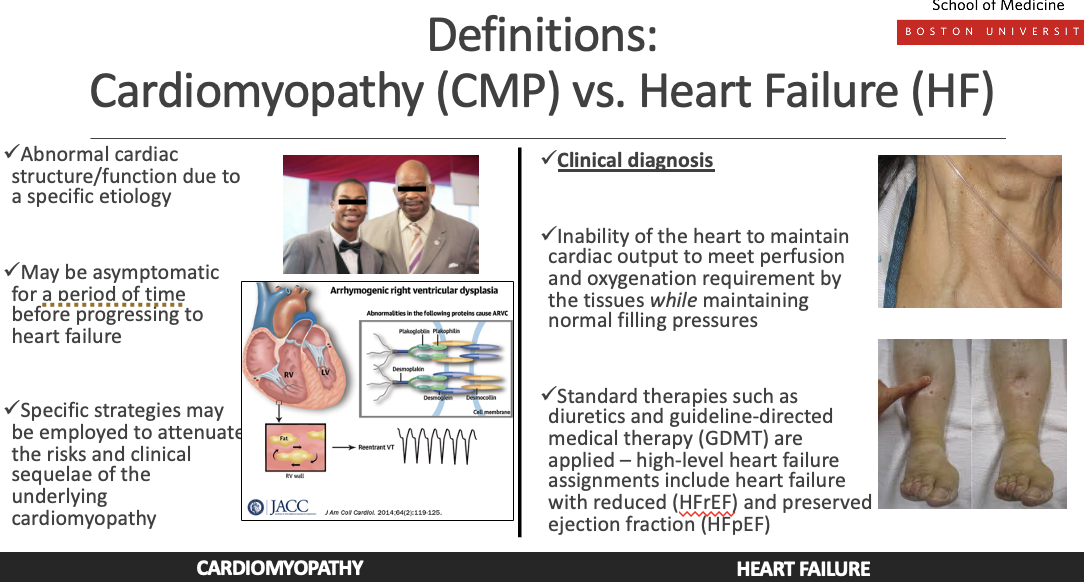
What is the differneeceb etwen cardiomyopathy and heat failuer
Cardiomyopathy is a disease of the heart muscle that causes structural or functional abnormalities (e.g., dilated, hypertrophic, restrictive).
Heart failure is a clinical syndrome where the heart cannot pump enough blood to meet the body’s needs, often resulting from cardiomyopathy or other heart diseases.
Cardiomyopathy (CMP)
Abnormal cardiac structure and function due to a specific etiologuy
May be asymptomatic for a period of time before progressing to hf
Specific strategies may be employed to attenuate the risks and clinical sequale of the underlying cardiomyopathy
Clinical diagnosis *heart faleure)
Inablituy to maintain cardiac output to meet perfusion and oxygenation requirement by tissues while maintaining normal filling pressure
Standand therapies such as diuretics and guideline-directe medical therapy (GDMT) are applied- hgih lelved heart faieure assingemnts include heart fialure with reduced ejection fraction and preserved ejeciton fraction
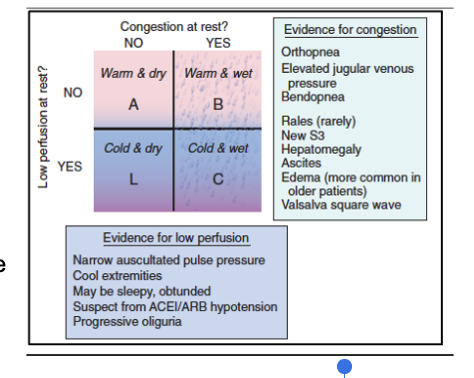
How do we diagnose hf. congestion vs low perfusion (note the same approach is used for hfpef and hfref)
This approach classifies heart failure patients based on volume status (congestion) and cardiac output (perfusion) using bedside clinical findings.
The Two Axes
Congestion: “Wet” (fluid overloaded) vs “Dry” (no congestion)
Perfusion: “Warm” (adequate perfusion) vs “Cold” (poor perfusion)
Four Hemodynamic Profiles
Warm & Dry
Adequate perfusion, no congestion
Compensated HF (goal state)
Warm & Wet (most common)
Adequate perfusion with congestion
Symptoms: dyspnea, edema, crackles
Treat with diuretics ± vasodilators
Cold & Dry
Poor perfusion without congestion
Symptoms: fatigue, cool extremities, hypotension
Treat with fluids or inotropes
Cold & Wet (worst prognosis)
Poor perfusion and congestion
Symptoms: shock, severe dyspnea, edema
Treat with diuretics, inotropes, ± vasopressors
Why It’s Used
Provides a rapid bedside assessment
Guides treatment decisions
Predicts prognosis
🧠 Quick memory aid:
*Wet = congestion; Cold = low output; Warm & Dry = winning combo.
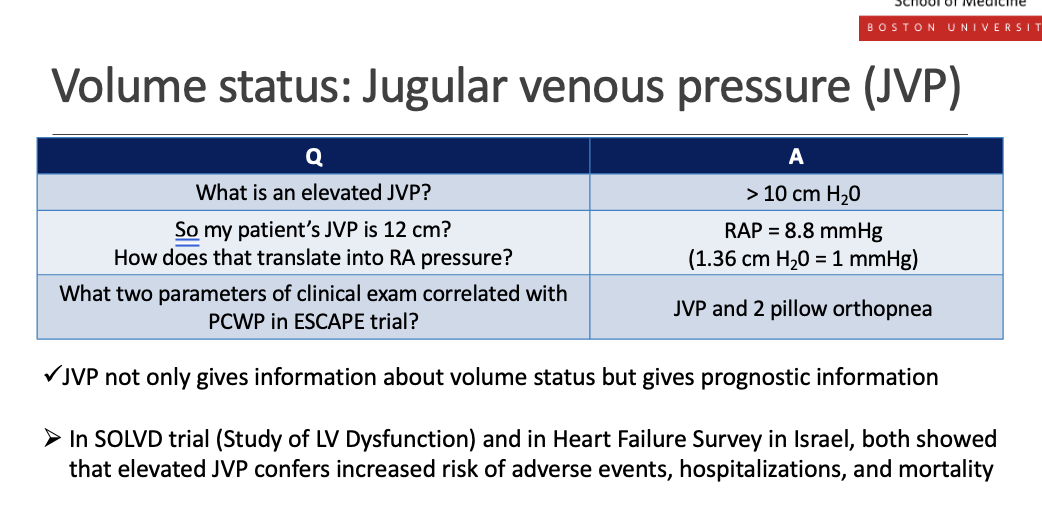
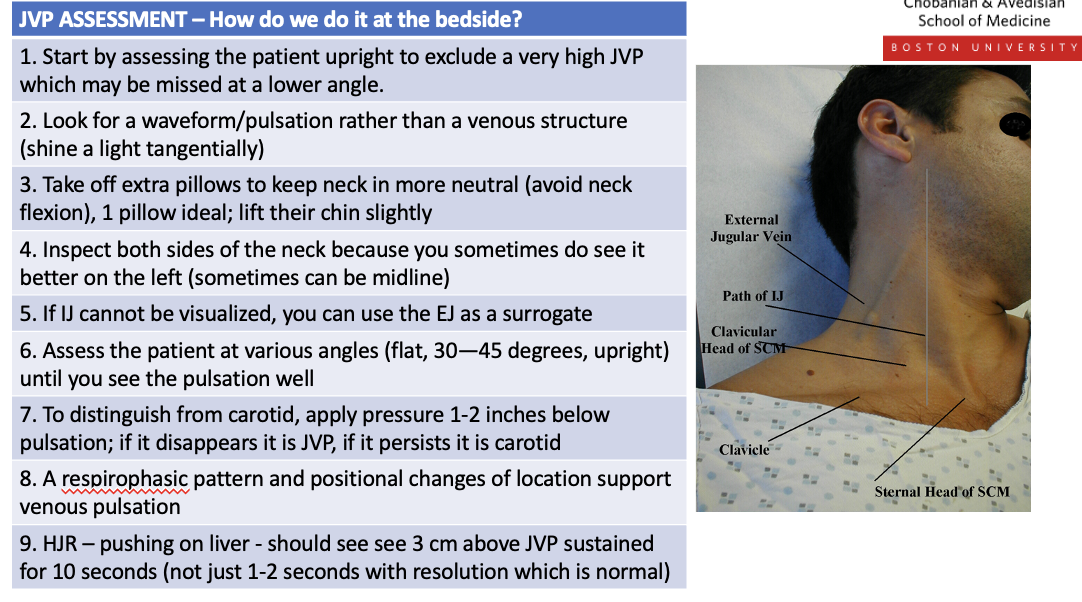
What are teh ssteps to a jvp assesment
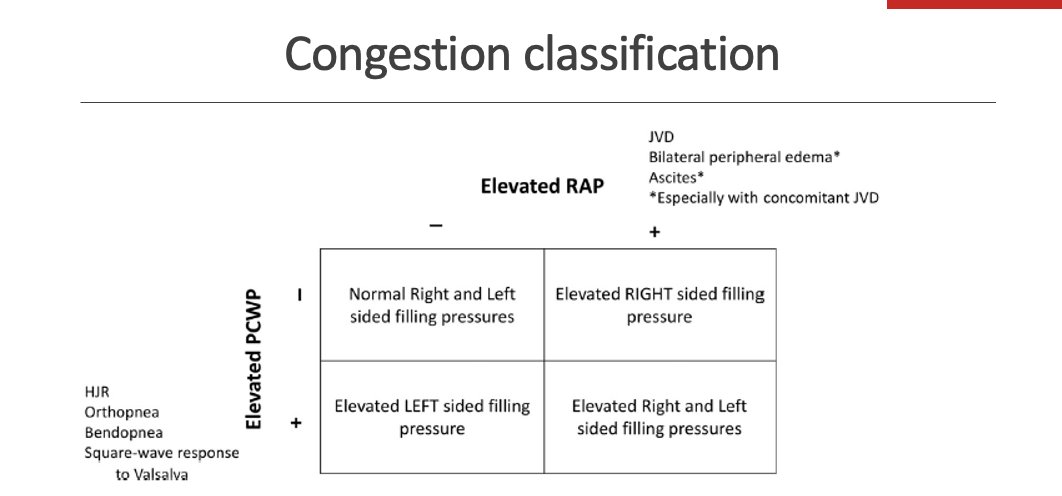
how would you clasify congesiton clinicaly using elevate pcwp and elevated rap
Pulmonary congestion (Left-sided congestion)
Elevated PCWP (pulmonary capillary wedge pressure)
Reflects increased left atrial pressure
Clinical signs/symptoms:
Dyspnea, orthopnea, paroxysmal nocturnal dyspnea, pulmonary crackles, cough
2. Systemic congestion (Right-sided congestion)
Elevated RAP (right atrial pressure)
Reflects increased systemic venous pressure
Clinical signs/symptoms:
Peripheral (pitting) edema, jugular venous distension (JVD), hepatomegaly, ascites
3. Biventricular congestion
Elevated PCWP and elevated RAP
Combined pulmonary and systemic congestion
Clinical signs/symptoms:
Dyspnea plus edema, JVD, ascites
🧠 Key takeaway:
PCWP ↑ = lung (left-sided) congestion
RAP ↑ = body (right-sided) congestion
Describe the valsava squre menuver in diagnosis. whata re teh steps. waht is the square
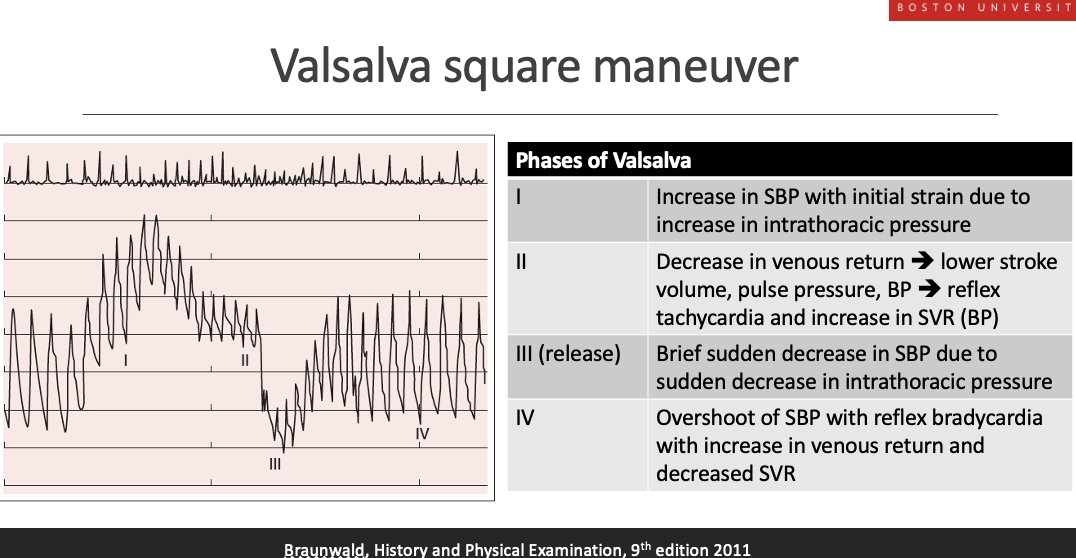
what is thesqare
•A ”positive square” has been shown in several studies to have excellent correlation with elevated PCWP (≥ 18 mmHg) (rho correlations 0.75-0.90)
•
During normal Valsalva, BP falls then recovers
In heart failure with high filling pressures, BP does not fall and instead stays elevated, producing a “square wave” arterial pressure tracing
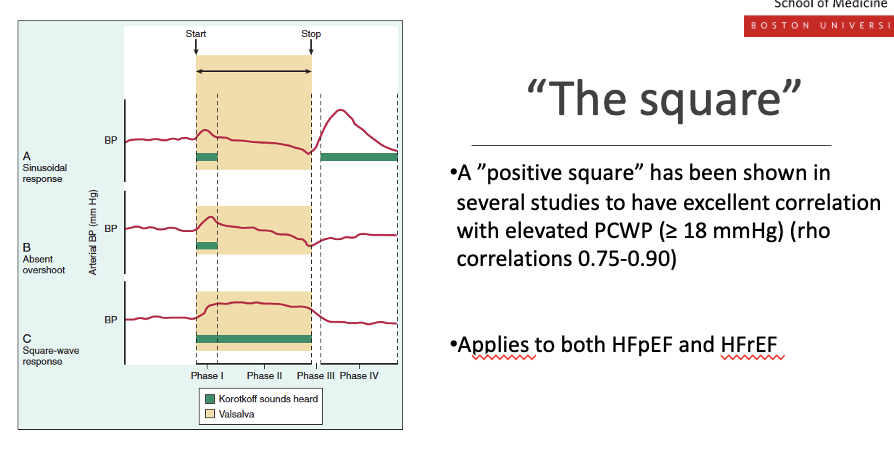
•Applies to both HFpEF and HFrEF
What is the use of swan-gantz catherders
What it measures
Right atrial pressure (RAP) → preload/systemic congestion
Right ventricular pressure
Pulmonary artery pressure (PAP)
Pulmonary capillary wedge pressure (PCWP) → left atrial pressure / pulmonary congestion
Cardiac output (thermodilution)
Mixed venous oxygen saturation (SvO₂)
Clinical uses
Diagnose and classify heart failure hemodynamic profiles
Differentiate cardiogenic vs noncardiogenic shock
Assess volume status and perfusion
Evaluate pulmonary hypertension
Guide therapy in severe or refractory HF
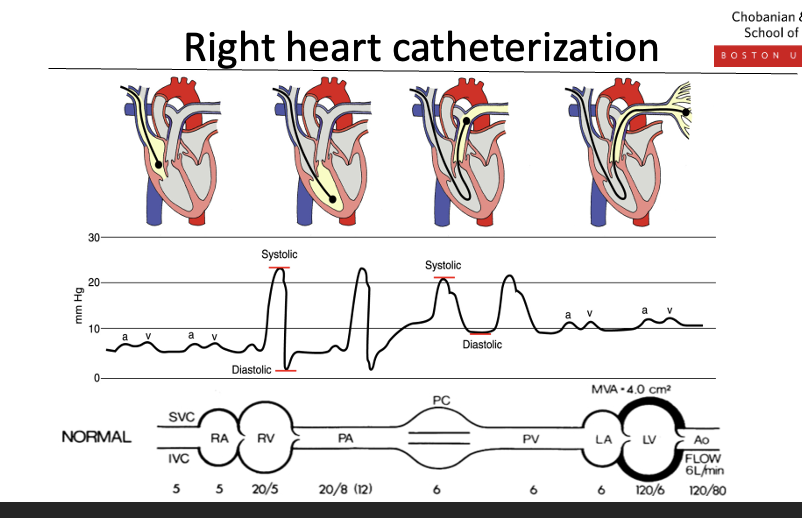
Relation to right heart catheterization
Right heart catheterization is the procedure
Swan–Ganz catheter is the tool used to perform it
The catheter is advanced through the right atrium → right ventricle → pulmonary artery, allowing measurement of pressures along the way
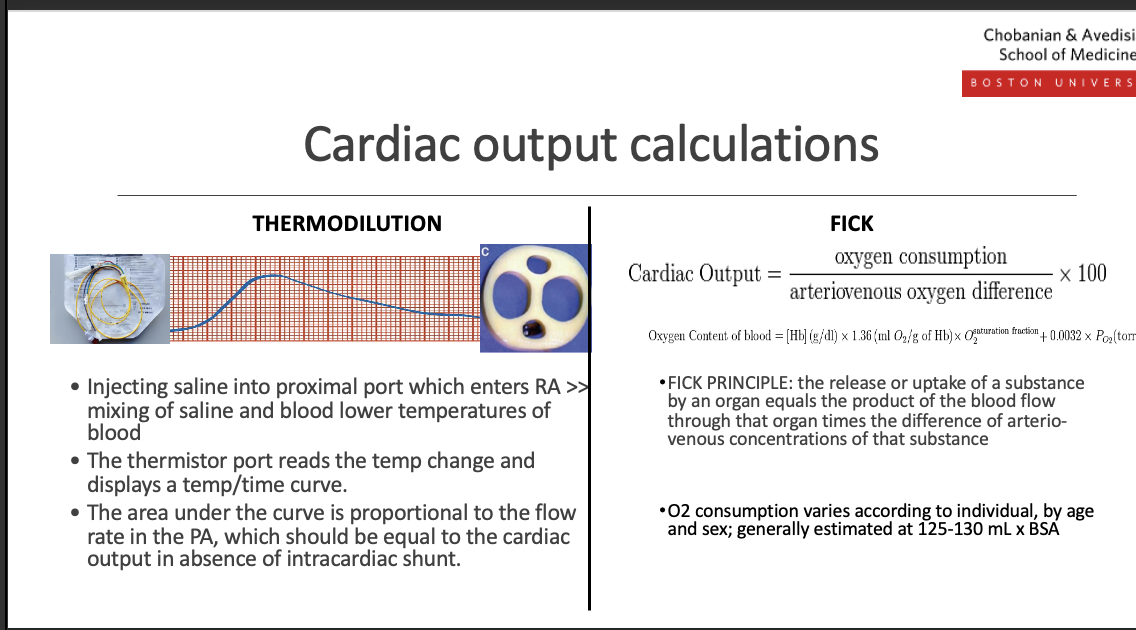
what are the cardiac output calculations (fick and thermomodulation and what is its use
Cardiac Output (CO):
CO = Heart Rate × Stroke VolumeThermodilution (Swan–Ganz):
CO is calculated by injecting cold saline into the right atrium and measuring the temperature change in the pulmonary artery; a smaller temperature change = higher CO, larger change = lower CO.Fick Equation:
CO = O₂ consumption ÷ (arterial O₂ − venous O₂)
Uses the difference between arterial and mixed venous oxygen content to calculate CO.
Key:
Thermodilution → invasive, catheter-based
Fick → based on oxygen delivery and consumption
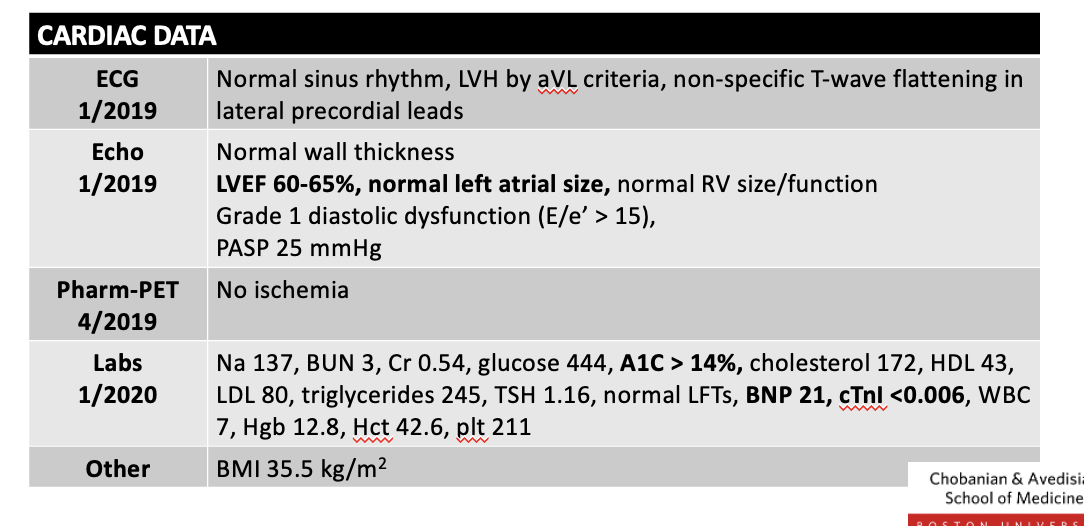
if possible analize this case study. do they hae hf
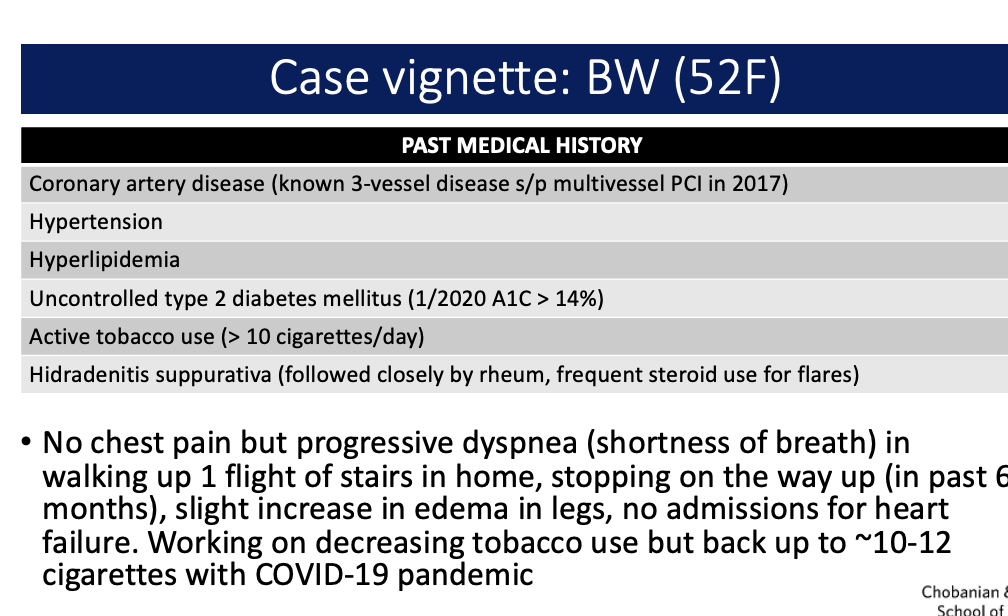
jjjj
What are the teraputic methods for hfpef
Diuretics:
Mainstay for symptom relief by reducing volume overload and congestionSGLT2 inhibitors (dapagliflozin, empagliflozin):
Reduce HF hospitalizations and mortality; benefit patients with or without diabetesBlood pressure control:
Use ACE inhibitors, ARBs, or ARNI to reduce afterload and LV stiffnessMineralocorticoid receptor antagonists (MRAs):
May reduce hospitalizations and improve symptoms in selected patientsRate and rhythm control (especially AF):
Beta-blockers or nondihydropyridine CCBs to improve diastolic filling timeTreat comorbidities:
Manage hypertension, obesity, diabetes, sleep apnea, CADLifestyle interventions:
Exercise training, sodium restriction, weight management
what are some of the theraputic methos for hfref
ACE inhibitors / ARBs / ARNI (sacubitril/valsartan):
Reduce mortality, hospitalization, and afterloadBeta-blockers (e.g., carvedilol, metoprolol succinate):
Improve survival, reduce arrhythmias, and improve remodelingMineralocorticoid receptor antagonists (spironolactone, eplerenone):
Reduce mortality and hospitalizationsSGLT2 inhibitors (dapagliflozin, empagliflozin):
Reduce hospitalizations and mortality, even in non-diabetic patientsDiuretics:
Symptomatic relief of congestion and edemaDevice therapy:
ICD: Prevent sudden cardiac death
CRT: For patients with wide QRS and LV dyssynchrony
Lifestyle interventions:
Sodium restriction, fluid management, exercise, weight controlAdvanced therapies:
Heart transplant or LVAD in refractory HFrEF
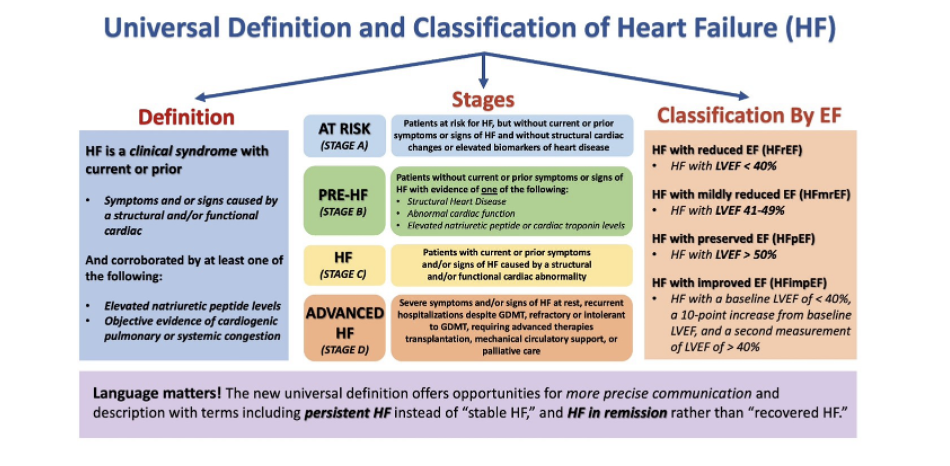
What is the universial definition and classifcaiton of hf chart and undeerstand it. what is at risk, prehf, hf ,advanced. waht is its stages, what is its definition, how do you classify
What is the fuction of GABA on stress funcrioning and exrcises
it is an inhibitory neuro transmitter that reduces stress siganling by blockign the pvn in the hypothalmus = CRTH is not released from the. hypothalamus = stress hormones not produced
= helps calm the nervous system =
Does swimming induce gene activation changes? why or why not
the aerobic and anarobic properites of experices cancel eachother out = you seeno gene activation patters of fao or glucose metabolism
what is the use of PGC1 -alpha
used in the mitochondria = increase of FFA acid use and beta oxidation = more ATP use
What is the difference between mitochondrial energy use for pathologic adaptations and physiologic adaptaion
patholodic remodeling
biogenisis, FAO
physilogic
adaptive hypertrophy
presered functioning
increased biogenesis, FAO