Parenteral Therapy
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
Aseptic technique
One which is designed to prevent contamination of materials, instruments, utensils, containers, during handling
Laminar Air Flow Hoods = Constant flow of HEPA filtered air at a rate of approx. 0.45 m/s
HEPA filter = High Efficiency Particulate Air filter removes 99.97% of all air particles 0.3 mm or larger
Critical Work Surface = the space between the HEPA filter and sterile product being prepared
Wash hands
At least 30 seconds
Scrub nails, hands and forearms
CDC allows you to use sanitizer on hands if they’re not soiled.
Enteral
Of or related to the intestines
(PO, Orally, per tube)
Parenteral
Introduced other by way of the intestines
(IV, IM, SubQ)
Laminar Air flow hoods
Horizontal flow (laminar flow hood)
Air blows towards worker
Used for non-chemotherapy preparations
Place items in a way so that airflow isn’t blocked
Have to work at least 6 inches in within the hood and can’t rest your arms on it
Should be run continuously but need to run it for 30 mins before use (if turned off)
Clean back to front away from HEPA filter
Don’t touch the HEPA filter, if you suspect injury to the filter shut off the hood and have someone suspect it
Vertical flow (Biological safety cabinet or chemotherapy hood)
Air blows from top down to maintain sterility and protect the worker
Used to make chemotherapy
What part of the hood is the most critical part to keep clean
HEPA filter
Barrier isolator technology
Closed system: workers manipulate compounding through gloved ports
Persoannel are the primary source of contamination of compounded preparations
Barrier isolator removes personnel from environment where parenteral products are prepared
*Good Aseptic technique is still required
Although they offer flexibillity for placement but USP <797> still requires proper technique and cleaning protocols
Syringes
Never touch the tip or plunger
Available in sizes ranging from 1 to 60 mL
DO NOT use syringes whose graduations are greater than twice the volume being measured
Ex: Don’t measure 5 mL in a 20 mL syringe
Needles
Hub:
Where the needle attaches to the syringe tip and allows the fluid in the syringe barrel
Bevel:
The tip of needle is slanted to a point and the slanted part of the needle is the bevel
The bevel allows for smooth insertions through stoppers and ports with minimal coring
Coring:
The development of a core or hole in the rubber of a vial
To prevent coring insert needle at an angle
Insert bevel tip first, then pressing downward and toward the bevel so the bevel tip and heel enter at the same point
Common needle sizes
16 G and 1 ½ in
18 G and 1 ½ in
20 G and 1 in
(20 is finer than 16)
Recapping needles:
Use on hand “scoop the cap” technique
797 discourages recapping only if required for sterility or safety
Packaging
Single dose: Hermetic container holding a quantity of sterile drug intended for parenteral administration as a single dose
Ex: Ampuls sealed by fusion
Multiple dose: Hermetic container permits withdrawal of successive portions of the contents without changing the strength, quality, or purity of the remaining portion
Vials and Ampules
Swab rubber closure with 70% alcohol using firm strokes in the same direction
To prevent vacuum formation:
Iiject an equal amount of air for the volume of fluid to be removed
Reconstituting drug powder:
Remove an equal amount of air for the volume of diluent added
To break ampule:
Clean ampule neck with alcohol swab
Leave swab in place
Grasp ampule neck with thumb and index finger
Use quick, firm snapping motion away from body and HEPA filter towards side wall of hood
Packaging
Open at the edge of the hood so as to not introduce contamination to the hood
Labeling
Name of product
% of drug or amount of drug in specified volume of amount of drug and volume of liquid to be added
Manufacturer/Distributor
Lot number
Name and quantity to be added
Immediate use CSPs
When all of the following conditions are met, compounding of CSPs for direct and immediate administration is not subject to the requirements for Category 1, Category 2, or Category 3 CSPs:
Aseptic techniques, processes, and procedures are followed, and written SOPs are in place to minimize the potential for contact with nonsterile surfaces, the introduction of particulate matter or biological fluids, and mix-ups with other conventionally manufactured products or CSPs.
Personnel are trained and demonstrate competency in aseptic processes as they relate to assigned tasks and the facility’s SOPs.
The preparation is performed in accordance with evidence-based information for physical and chemical compatibility of the drugs (e.g., approved labeling, stability and compatibility studies).
The preparation involves not more than 3 different sterile products.
Any unused starting component from a single-dose container must be discarded after preparation is complete.
Single-dose containers must not be used for more than one patient.
Administration begins within 4 h following the start of preparation. If administration has not begun within 4 h following the start of preparation, it must be promptly, appropriately, and safely discarded.
Not for high-risk or hazardous drugs
Preparing a product per approved labeling
Preparing a conventionally manufactured sterile product in accordance with the directions in the manufacturer’s approved labeling is out of scope of this chapter only if
The product is prepared as a single dose for an individual patient; and
The approved labeling includes information for the diluent, the resultant strength, the container closure system, and storage time.
CSP Category 1
CSPs are compounded under the least controlled environmental conditions and therefore are assigned a BUD of 12 h or less at controlled room temperature or 24 h or less when refrigerated, if compounded in accordance with all of the applicable requirements for Category 1 CSPs in this chapter
CSP Category 2
CSPs require more environmental controls and testing than Category 1 CSPs and may be assigned a BUD of greater than 12 h at controlled room temperature or more than 24 h if refrigerated, but not exceed the limits established in Table 13 if compounded in accordance with all of the applicable requirements for Category 2 CSPs in this chapter.
CSP category 3
CSPs undergo sterility testing, supplemented by endotoxin testing when applicable, and have more requirements than Category 2 CSPs for personnel qualification, use of sterile garb, use of sporicidal disinfectants, frequency of environmental monitoring, and stability determination. Category 3 CSPs may be assigned longer BUDs than those set for Category 2 CSPs but not exceeding the limits in Table 14 (see 14. Establishing Beyond-Use Dates), if compounded in accordance with all applicable requirements for Category 3 CSPs in this chapter (see 14.4 Additional Requirements for Category 3 CSPs).
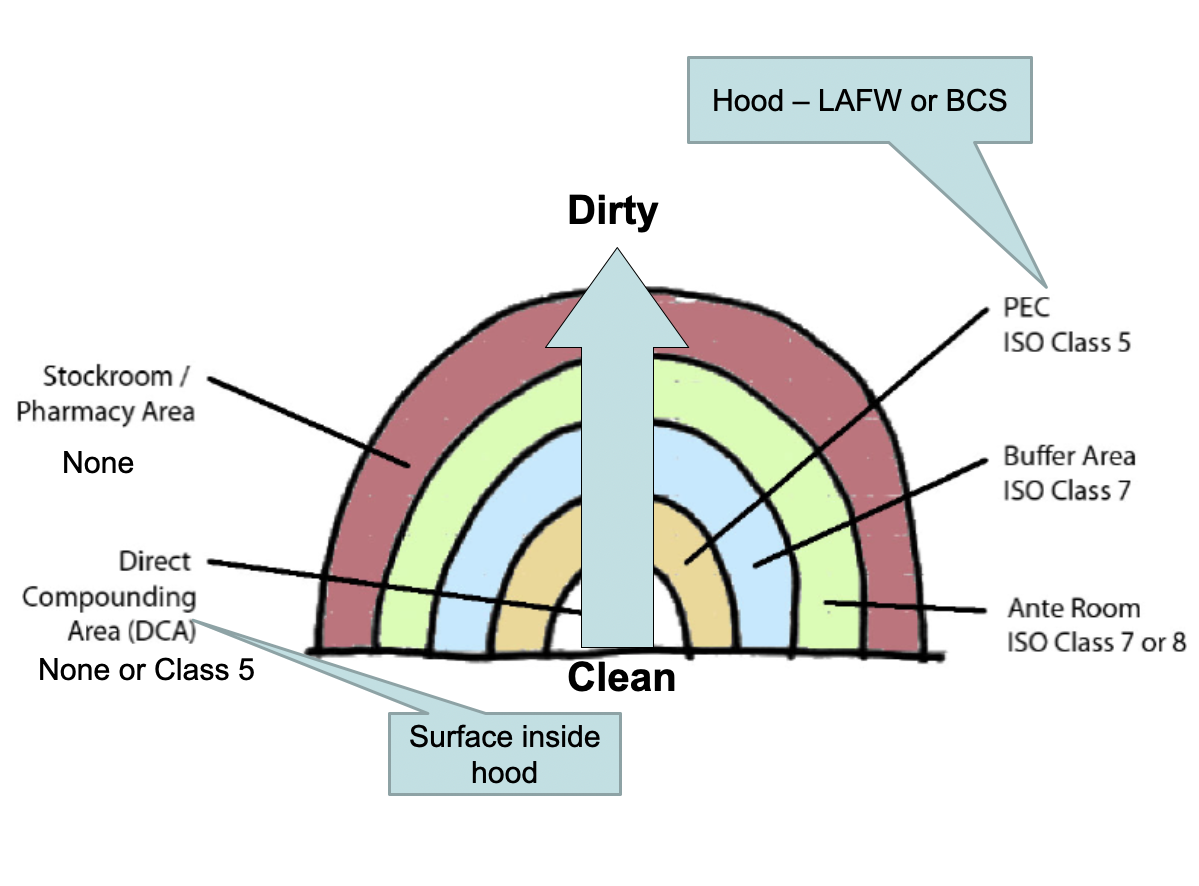
Environmental Quality and Control
Primary environmental areas are:
Direct Compounding Area (DCA) = surface in hood
Primary Engineering Control (PEC) = hood
Buffer Area and the Ante Area
The rest of the pharmacy
Goal is to eliminate the number of particles in each area by properly cleaning and garbing as you go from a dirty areas to cleaner areas.
Restricted access barrier system (RABs)
An enclosure that provides HEPA-filtered ISO Class 5 unidirectional air that allows for the ingress and/or egress of materials through defined openings
Compounding aseptic containment isolator (CACI)
A type of RABS that uses ONLY HEPA filtration to provide an ISO Class 5 unidirectional air environment for compounding sterile HDs
Compounding aseptic isolator (CAI)
A type of RABS that uses only HEPA filtration to provide an ISO Class 5 unidirectional air environment designed for compounding of sterile NON-HD
Facility design under 797
Must have an ISO Class 5 environment as a PEC for critical site exposure. Laminar airflow workbenches (LAFW), BSCs (biological safety cabinets), CAIs (Compounding Aseptic Isolator), and CACIs (Compounding Aseptic Containment Isolator) are common ISO Class 5 environments.
The compounding area must be separated from activities not essential to CSP preparation and must be a controlled (particle, temperature) environment.
Must have an ISO Class 7 environment for buffer area or cleanroom.
Must have an ISO Class 8 environment for ante areas.
Buffer areas physically separated from ante areas must have a positive pressure differential; if no physical separation is present, displacement airflow principles must be used (high airflow velocity, low pressure differential).
Must have adequate ACPHs (air change per hour) to maintain appropriate ISO Class, ACPH may be improved upon by PECs that re-circulate air between the room and the PEC itself.
Environmental Testing
Environmental monitoring must be routinely performed to prove that the compounding environment is properly maintained.
Must conduct regular surface sampling to test for adherence to cleaning and disinfecting procedures.
Cleaning procedures
There must be detailed cleaning and sanitizing procedures for ISO Class 5 PECs in order to maintain the cleanliness of the direct compounding area.
Personnel Cleansing and Garbing
Every institution must have a procedure for donning and doffing PPE for compounding
EX:
Remove personal outer garments (e.g., bandanas, coats, hats, jackets, sweaters, vests)
Remove all cosmetics because they shed flakes and particles
Remove all hand, wrist, and other exposed jewelry, including piercings that could interfere with the effectiveness of garbing (e.g., the fit of gloves, cuffs of sleeves, and eye protection) or otherwise increase the risk of contamination of the CSP. Cover any jewelry that cannot be removed.
Not wear earbuds or headphones
Not bring electronic devices that are not necessary for compounding or other required tasks into the compounding area
Keep nails clean and neatly trimmed to minimize particle shedding and avoid glove punctures. Nail products (e.g., polish, artificial nails, and extenders) must not be worn
Wipe eyeglasses, if worn
Labeling according to USP 797
The label on each immediate container of the CSP must, at a minimum, display prominently and legibly the following information:
Assigned internal identification number (e.g., barcode, prescription, order, or lot number)
Active ingredient(s) and their amount(s), activity(ies), or concentration(s)
Storage conditions if other than controlled room temperature
BUD
Dosage form
Total amount or volume if it is not obvious from the container
If it is a single-dose container, a statement stating such when space permits
If it is a multiple-dose container, a statement stating such
The labeling on the CSP must display the following information, as applicable:
Route(s) of administration
Special handling instructions
Warning statements
Compounding facility name and contact information if the CSP is to be sent outside of the facility or healthcare system in which it was compounded
Major Safety Requirements for Parenteral Products
Sterile products, aqueous solutions, emulsions and suspensions, are susceptible to microbial contamination
Sterility: Parenteral products must be free from microbial contamination, achieved by a comprehensive contamination control strategy
Pyrogen-free: Parenteral products must be free from pyrogens that cause fever and other adverse reactions
Particulate-free: Parenteral products must be free from visible and sub-visible particles that cause embolisms and other adverse reactions
Sterilization
Sterilization: Complete destruction of all living organisms and their spores or complete removal
Sterility assurance level (SAL): 1/106
Approaches
Initial control
Terminal sterilization (Product in primary packaging)
Aseptic processing and sterile filtration
Preservation (Antimicrobial agent)
Microbial Contamination of Parenterals
Strict adherence to good manufacturing practice (GMP) practices, including manufacturing, personal training, and facility design
Many parenteral products undergo terminal sterilization
Aerobic microbial growth is inhibited by N2 gas because it displaces O2
Immunocompromised patients are vulnerable to microbial contamination due to weakened immune systems.
Healthcare staff must be rigorously trained in proper aseptic technique for preparing and administering injections in clinical settings
Death rates
Demonstrates first order kinetics
D value – time in minutes required to destroy 90% of microbes under a standard set of conditions
D = 1 minute – Thus, 106 organisms goes to 105 after 1 minute
After 8 minutes, 10-2 survive
Biological Indicator
Specific microorganism resistant to a particular method of sterilization
Validation of sterility
Add at known level and known D value and sterilize – survival should be 10-6 or less
Steam Sterilization
Moist heat – Heating under pressure in the presence of water to generate steam
D = 1 min at 121 °C: USP: 120 °C at 15 psi for 20 min
Autoclaves
Moist heat causes irreversible denaturation (unfolding of proteins) of essential proteins
Metal needles, glass syringes, plastic bags, vial closures
Single dose solution in a sealed container, e.g., LVP, ampoule
Steam sterilization damages oils, proteins, and powders
Dry heat sterilization
Dry heat sterilization is done in sterilizing ovens
D = 5 min at 160 °C: USP: 160 to 170 °C for greater than 2 hours
Mechanism of microbial destruction is dehydration, denaturation, and slow burning or oxidation
Depyrogenation – Removal of pyrogens, fever-causing substance
Oils and fats, powders, glassware, and metal apparatus
Not suitable for plastics and rubber
Radiation Sterilization
Gamma radiation (Co-60)
15-50 kGy dose for sterilization
High penetration
Irradiation of an aqueous solution ejects electrons and creates free radicals that react with biological macromolecules, especially DNA, leading to cell damage and death
Irradiation of powders for injection, e.g., beta-lactams
Medical devices, e.g., plastic syringes, packaging materials, ointments, and implants
Ionizing radiation damages biologic drugs
Gas Sterilization
Some heat-sensitive and moisture-sensitive materials
Ethylene oxide (EO) gas is combined with heat 40 to 60 ◦C and moisture using specialized equipment
EO is an alkylating agent – it reacts amine groups of DNA
EO penetrates into hard-to-reach places in packaged items
Terminal sterilization – Residual EO is toxic and must be allowed to dissipate after sterilization and before use of sterile product
Used for Medical devices, e.g., plastic syringes, catheters, and certain heat-labile enzymes and certain antibiotics
Preservatives in parenteral products
Bacteriocidal agent that kills microorganisms
Bacteriostatic agent that prevents growth of microorganisms
Products may not require preservative due to inherent properties
Few microorganisms grow below a pH = 3 (Pickling)
Few microorganisms grow above pH = 9
Cosolvents, > 15% alcohol, may not require antimicrobial agent
Microorganisms cannot grow in hypertonic solutions, e.g., 67% sucrose in syrup
Single-dose preparations eliminate need for preservatives
Routes of administration
IV benzyl alcohol caused deaths of neonates given preserved saline – “Gasping Syndrome”
Intrathecal benzyl alcohol increased risk of neurological events
Epidural injection of benzyl alcohol is contraindicated
Preservative-free products are compounded by strict aseptic technique to maintain sterility
Preservatives in parenterals have broad spectrum antimicrobial activity against bacteria, yeasts, and molds
USP tests for Pyrogens
Limulus amebocyte lysate test
Horse-shoe crab
A sample is incubated for one hour at 37 °C with blood cells
A gel forms in the presence of pyrogen
Picogram (10-12 g)
Rabbit febrile reaction
Sample is injected into the ear vein of a rabbit, and the rectal temperature of the animal is taken over 3 hours
An elevation of > 0.6 °C above normal is the basis for failure of the test
About an hour after injection of pyrogen into rabbits (or man)
Sterile filtration
Physically removes microorganisms from solution using filters with small pore size
Preferred method for solutions that are unstable by thermal, chemical, or radiation sterilization, such as biologics
Microbial filter (0.2 μm), e.g., cellulose esters
Removes bacteria and molds
Does not remove not endotoxin, viruses
Drug loss by filtration, e.g., amphotericin B (Self-aggregation)
Drug adsorption on membrane, especially biologics at low concentration
Lipid nanoparticles (LNPs) can block or ”foul” filter membranes
Visual inspection of parenteral products
Bacteria/Fungi
Protein aggregation/ precipitation
Incompletely dissolved drug
Precipitate from incompatible admixtures
Glass particles from ampoules
Particles from rubber stoppers and plastic
Regulatory compliance
Instrumental or microscopic testing
Visual inspection of parenterals for particulates
100-150 μm
Black/White background
Color change is an important for quality control
Particle size effect on Parenterals
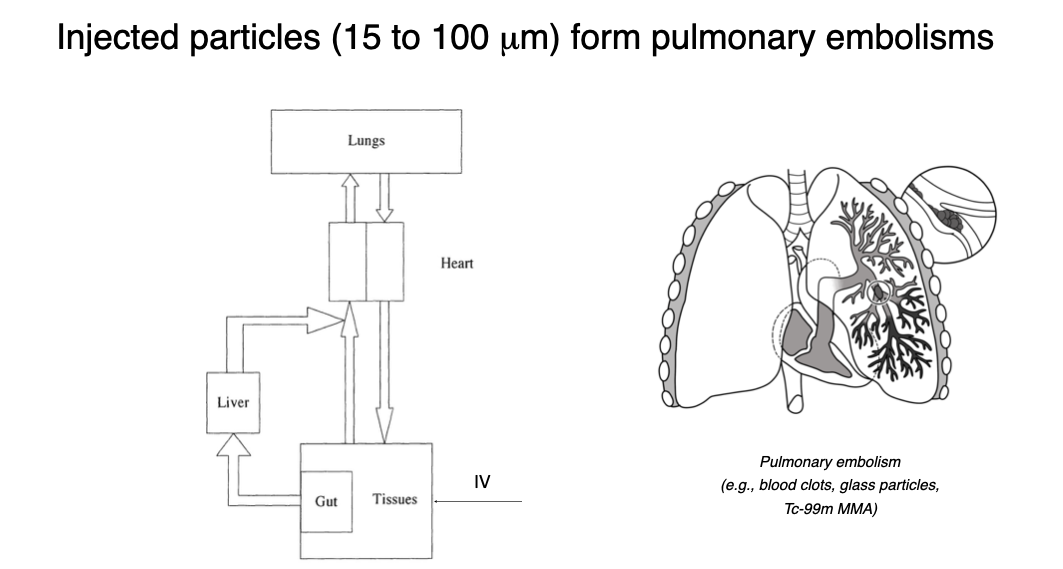
Parenterals must be free from visible and sub-visible particles that cause embolisms and other adverse reactions
Particles can be emboli, i.e., matter carried in blood, which can lodge in a blood vessel, obstructing it
Resulting blockage or occlusion is an embolism
Thromboembolism: Embolism of thrombus (Blood clot)
Particles can cause phlebitis
Mechanical irritation and inflammation of the vein
Pain and tenderness along course of vein
Incidence of phlebitis is reduced when IV infusions are filtered (Final filter)
Top 10 excipients in injectable products
Water
Sodium chloride
Tween 80
Mannitol
Propylene glycol
Benzyl alcohol
Citric acid
Sodium citrate
Glycine
Lactose
Major factors of microbial growth and death
Radiation
Temperature
Time of storage
Chemical (nutrient, pH)
Moisture content
Gaseous Environment
C0
*Hand contact is a primary way that bacteria are transmitted to catheters
Large volume IV solution
Also called large volume parenterals or LVPs, these are single‑dose injections containing more than 100 mL of solution that are intended for intravenous use
Small volume injection
These are Injections of 100 mL or less. They may be either single and multidose products
What to consider for Medication Administration?
Where is the location of the med being given
Volumes for each injection (and when to know its too much/too little volume)
What size syringe to use
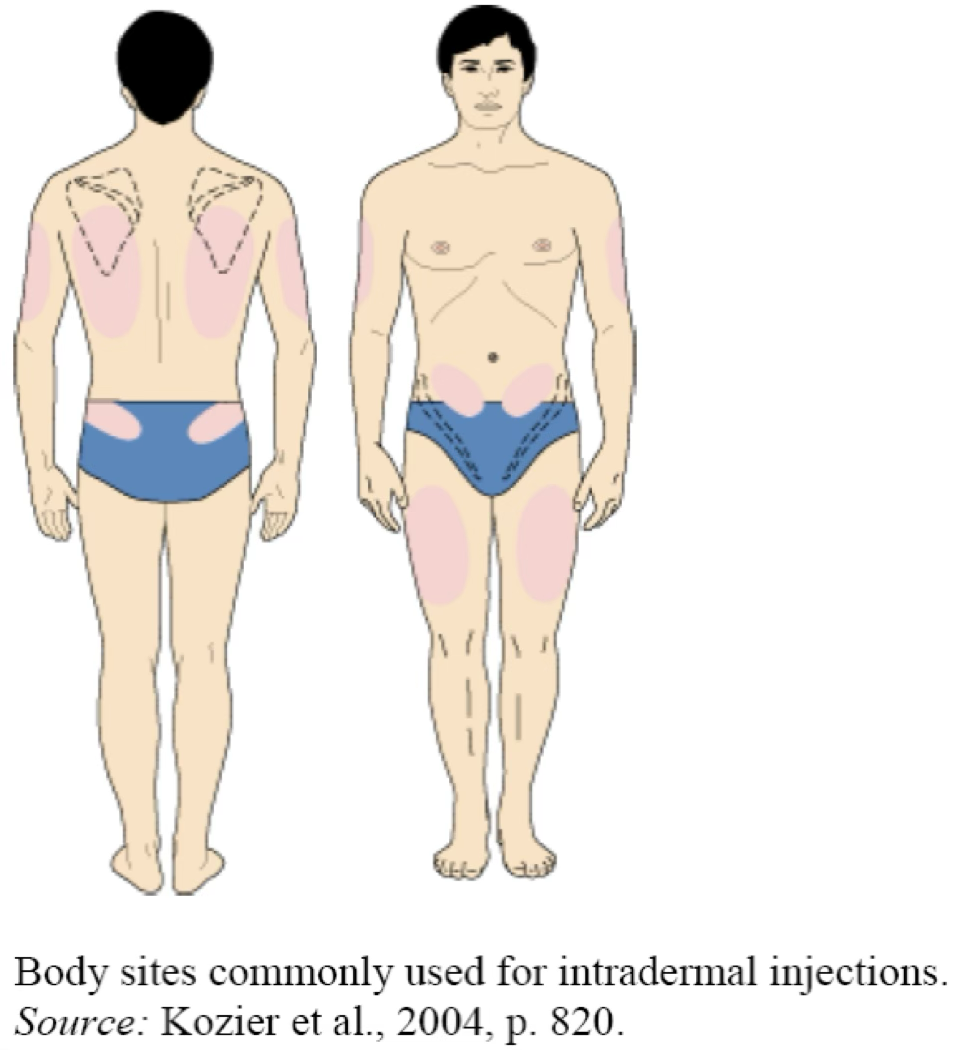
Intradermal
Injection area: Located just below the surface of the skin (at the interface between the epidermis and dermis). This route is most often used for skin tests in which systemic absorption is undesirable and could be dangerous (e.g., serious allergic reactions).
Volumes: Limited to small quantities, usually 0.1 mL, but may be as small as 0.02 mL and as large as 0.5 mL.
Syringes size: 1 mL syringes, often labeled "tuberculin" because these syringes were used to administer tuberculin skin tests.
Unlike other syringes, these are available with and without needles attached.
If a syringe is sent to a nursing unit with a needle attached, you must be certain the needle cover is snapped in place.
If it is not sent with a needle, the syringe should be filled with 0.1 mL excess for priming the new needle.
The syringe should then be labeled with a statement to this effect: "This syringe contains 0.1 mL excess for priming."
Needle Sizes: 25–28 gauge, 3/8 to 5/8 inch length
Subcutaneous
Injection area: Subcutaneous fat tissue located beneath the skin between the dermis and muscle. When administering a drug subcutaneously, the skin may be pinched up to avoid giving the drug into the muscle. This route is used for insulin, injectable pain medication, and others where specified.
Rotation of injection sites to avoid lipodystrophy.
Volumes: Limited to approximately 1 mL
Syringes sizes: 1 or 3 mL
Needle sizes:
This depends on use. Insulin syringes have ultrafine needles of 30 gauge (a 32 gauge needle is planned), ½ inch, "hypos" are often 25 gauge, ½ to 5/8 inch length.
Insulin syringes come with the needle attached. It is left in place for administering the dose after withdrawing the drug from the product vial.
For other drugs, the needle used for withdrawing the dose is usually removed; a Luer tip cap is applied; and the nurse or caregiver selects and attaches an appropriate needle at the time of injection.
In this case, excess for priming the new needle should be included in the syringe and the syringe labeled to this effect.
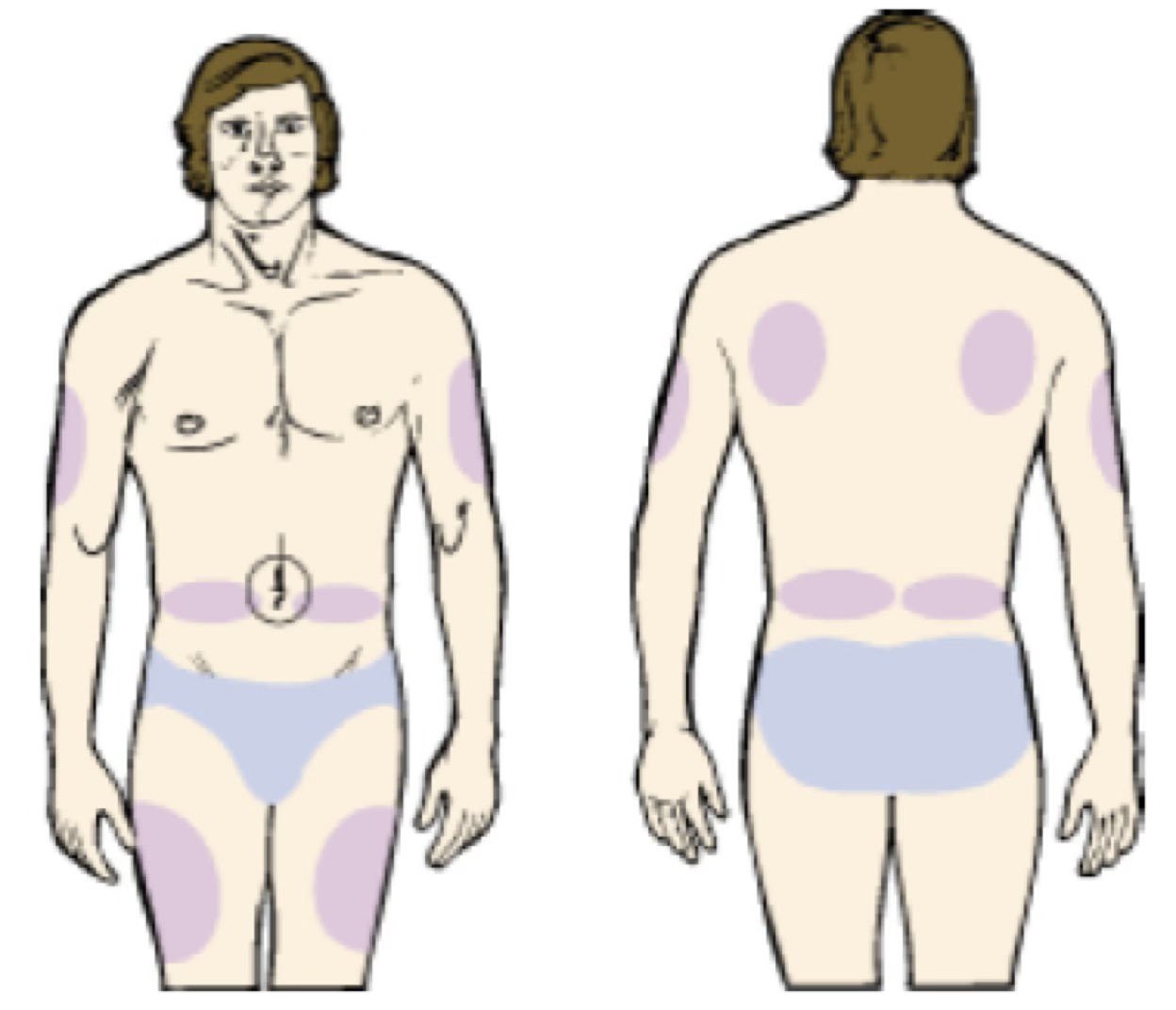
Intramuscular
Injection area: Muscle mass—deltoid (arm), gluteus maximus (buttocks), vastus lateralis (top of leg). Any nonirritating drug can be given by this route.
Syringes sizes: 1–5 mL
Needle sizes: 20–22 gauge, ½–1 ½ inches in length.
Aspiration is no longer recommended for most IM injections (per CDC).
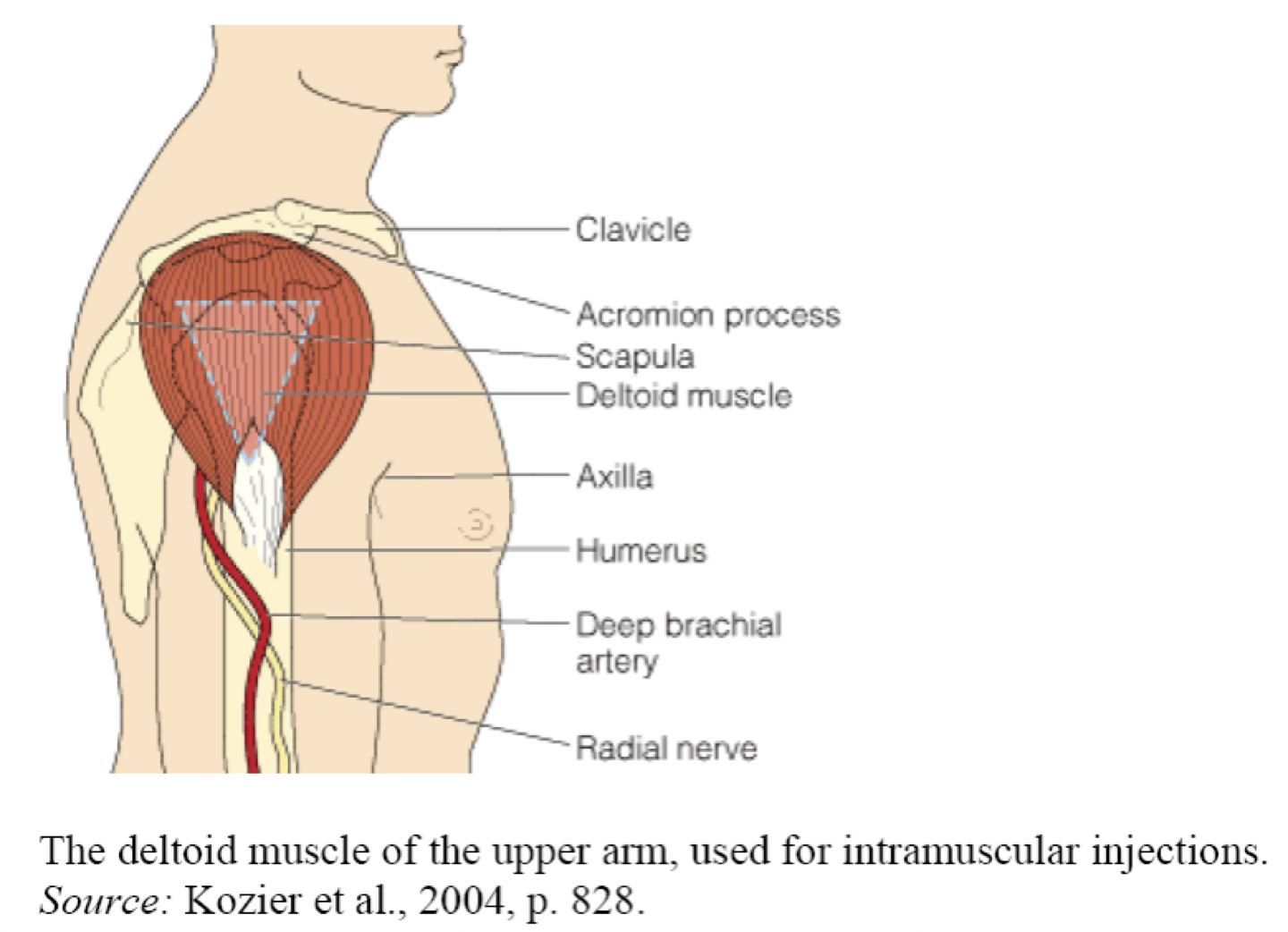
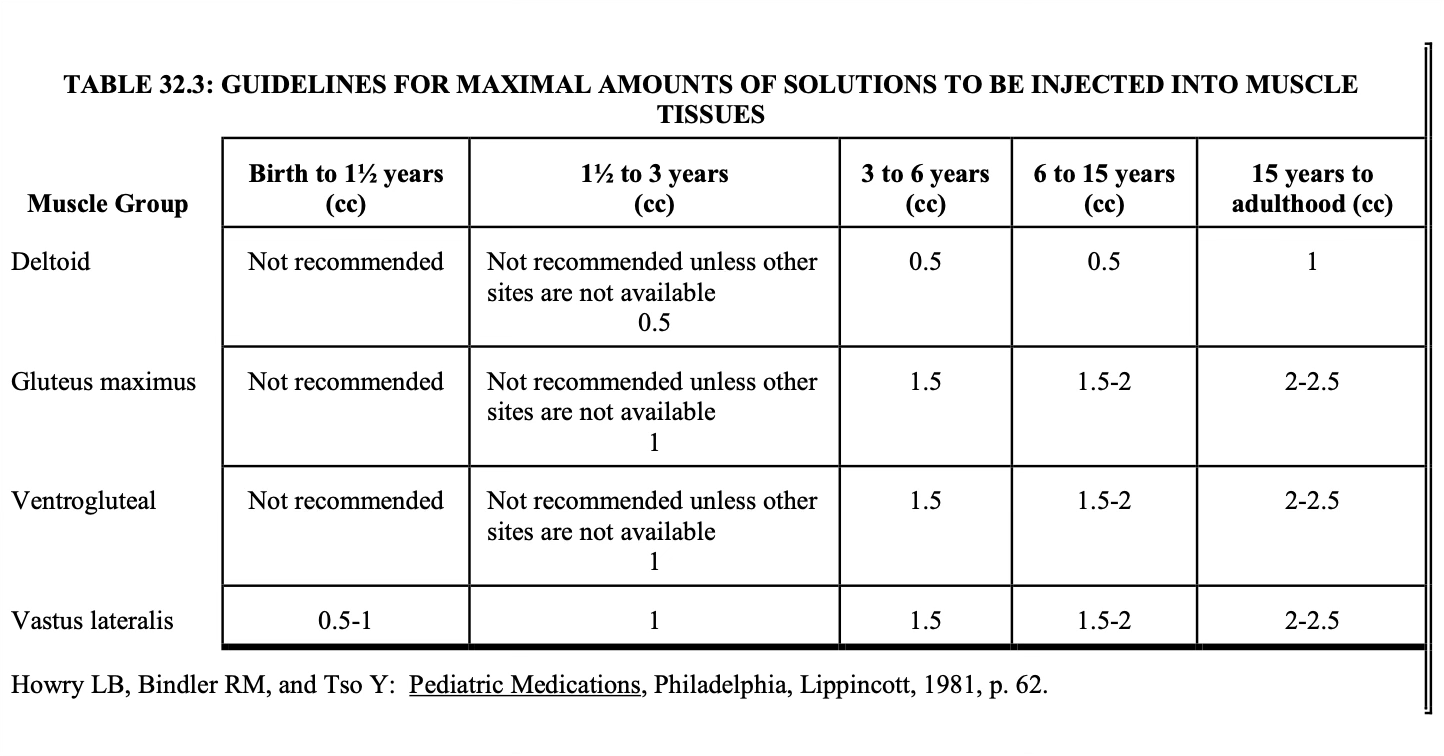
IM maximal volumes
The volume administered is limited by the mass of the injected muscle.
For adults, up to 2 mL may be given in the deltoid muscle of the upper arm, and up to 5 mL into the gluteal medial muscle of the buttock (these upper limits may be painful).
For children, the volumes are more restricted.
Notice that for small children, the vastus lateralis muscle is the recommended muscle because it is the largest muscle mass in children under 3 years of age and it is free of major nerves and vessels.
The gluteus maximus is not well developed until a child has walked for at least 1 year. It is also avoided because it has a major large nerve, the sciatic nerve, running through the middle.
Notice that for children up to 3 years, the maximum volume is 1 mL.
Be sure to keep this in mind when making IM injections for children.
Intravenous
Injection areas: Veins. This route is used for fluid, electrolyte, and nutrient replacement; for administration of any drug that needs to get into the circulation immediately; for irritating drugs; and for drugs that require carefully controlled blood levels.
Volumes: Obviously, volume is less of a limitation with intravenous therapy.
There are fluid restrictions—about 3 liters a day for adults and less for children.
Certain disease states further restrict fluid load.
The flow rate may also be restricted by the size of the vein chosen for administering the drug.
Syringe sizes: 1–60 mL
Needle: 20–22 gauge, ½–1 ½ inches in length.
Intravenous administration is further subdivided into Continuous or Constant Infusion, Intermittent, and Bolus or IV Push.
IV continuous
The drug is added to a large volume parenteral solution, and the solution is then slowly and continuously dripped into a vein.
Advantages:
It allows fluid and drug therapy to be administered simultaneously.
It achieves continuous, constant blood levels for the drug.
It minimizes vein irritation and trauma because most drugs are less irritating when in dilute solutions.
Continuous infusion is usually a cost‑saving over intermittent or bolus administration because fewer units are needed, and less nursing and pharmacy staff time is involved in preparation, processing, and administration.
Disadvantages:
The IV requires greater monitoring because it runs continuously.
If the IV infiltrates and cannot be continued, part of the dose has not been administered.
It cannot be used on fluid‑restricted patients.
The extended run times cannot be used with certain unstable drugs.
Intermittent
The drug is added to an intermediate volume (25–100 mL) and given in an intermediate period of time (15–60 minutes), at spaced intervals, such as every 6 hours
Advantages:
It requires less monitoring than continuous infusion.
The complete dose is given in a moderate fluid volume and over a moderate period of time; therefore, there is less chance than with bolus administration of toxicity without the disadvantages of continuous administration.
Many drugs are more stable at moderate concentrations than in the concentrated solutions required by bolus administration.
Disadvantages:
Fluids and some electrolytes cannot be given this way.
Drug blood levels are less constant than with continuous administration.
The method cannot be used for direct administration to an organ or tissue.
It is sometimes impractical for immediate injection in emergency situations.
IV bolus
The drug solution is placed in a syringe and administered in a short period of time (minutes) directly into a vein or IV tubing that goes into a vein. This may be a one‑time administration or it may be repeated at spaced intervals.
Advantages
It can be used for immediate injection in emergency situations.
It requires no monitoring of IV fluid administration.
It is less expensive than intermittent administration because there is no extra IV tubing or bag.
Disadvantages
Many drugs are more irritating in these highly concentrated solutions.
Some drugs are less stable in concentrated solutions.
Drug toxicity is a greater problem when a total dose is given in a bolus over a short period of time.
Drug blood levels are less even than with either continuous or intermittent IV dosing.
When repeated doses are given, it may require more staff time because at least 2–10 minutes may be needed at the bedside for each dose given.
Five Rights
Right medication. Am I administering the correct/intended medication?
Right dose. Am I administering the right amount of the medication? Is the medication’s concentration correct? Is the rate of administration correct?
Right route. Am I administering the medication via the right route? (Some medications, when delivered by the wrong route, have much different effects than intended.)
Right patient. Am I administering the medication to the patient who needs it and for whom it is intended?
Right time. Will the patient benefit from this medication at this particular place and time? Is this medication clinically indicated for the patient’s current condition?
Priming volume
The extra volume of each drug solution that must be drawn to fill the hub of the syringe and to prime the new needle that the nurse will apply when administering the drug solution to the patient
If the drug volumes are equal, the priming volume may be split equally, one‑half for each drug. For example, if there are two drugs, and the desired priming volume is 0.1 mL, you would use 0.05 mL of each for the priming volume.
If the drug volumes are unequal, the priming volume must have the same proportion of each drug solution as is in the dose volume
Exception tuberculin syringes …. No priming volume
On label make sure you indicate that syringe contain priming volume
Guidelines for labeling parenteral products for inpatients
Labels for parenteral products should be affixed on the container such that the contents can be inspected, the volume can be identified, and the capacity markings can easily be read.
For large volume parenterals, the name, type of solution, and lot number on the manufacturer's label should be visible.
The label should be positioned so that it can be read as the product is being administered.
For example, for large volume parenterals and piggy‑back bags, the label should be upright when the IV is hanging.
Always put the total volume on the label