Chemistry: Phase Changes
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
solid to liquid
melting
liquid to solid
freezing
liquid to gas
vaporization
gas to liquid
condensation
solid to gas
sublimation
gas to solid
deposition
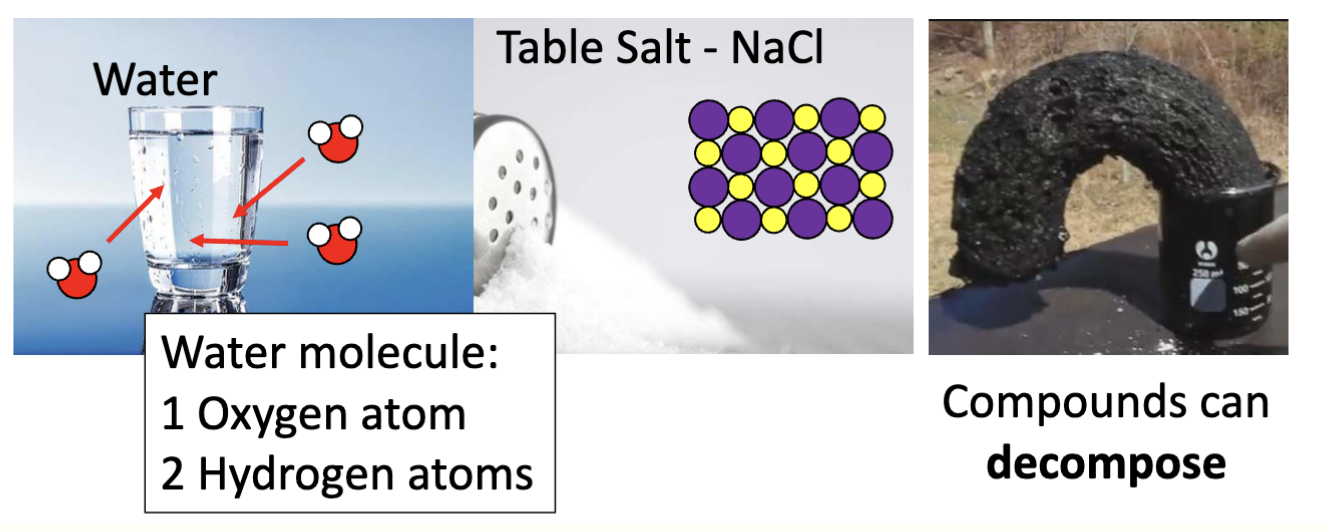
a substance made up of two or more elements in FIXED positions
compound
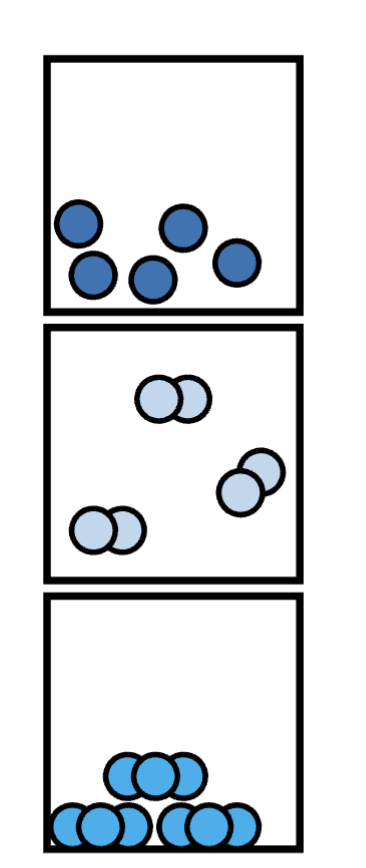
made up of only one particle
pure substance
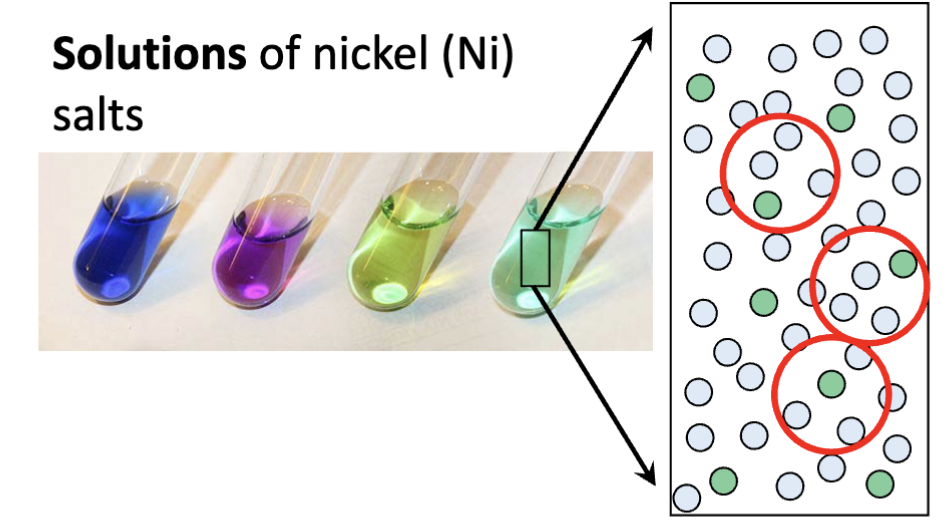
the composition of the mixture is uniform throughout the substance
Homogenous Mixture
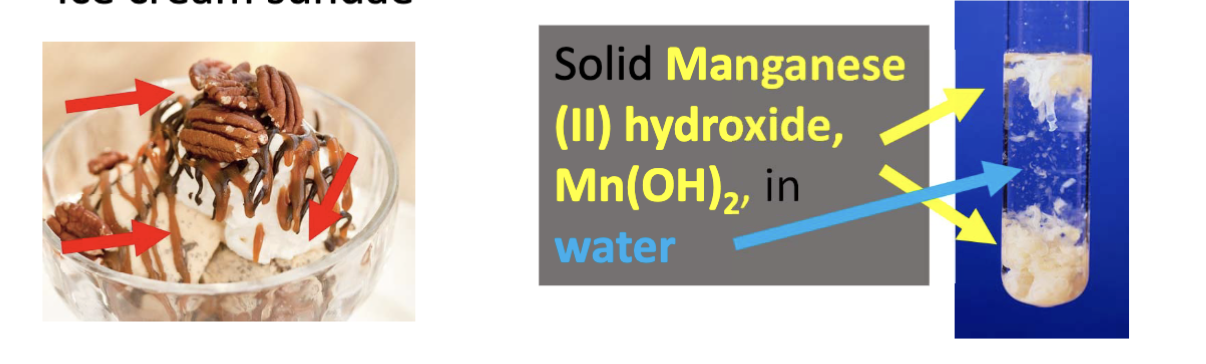
the composition of the mixture is not uniform throughout the substance and you can see the mixture with your eyes
heterogenous mixture

the conversion of a new substance to a new substance via a chemical reaction
chemical change

appearance changes but particles are the same as they were before
physical change
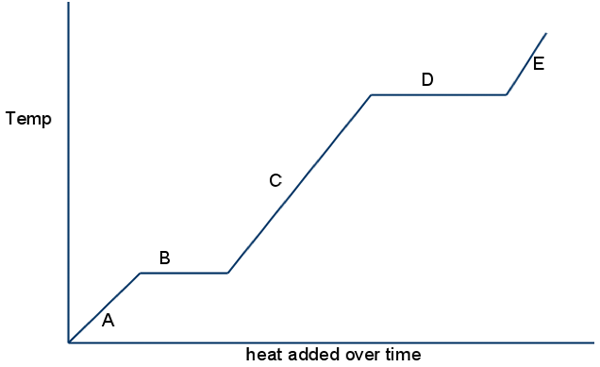
Look at B & D, what happens to potential energy?
potential energy goes up
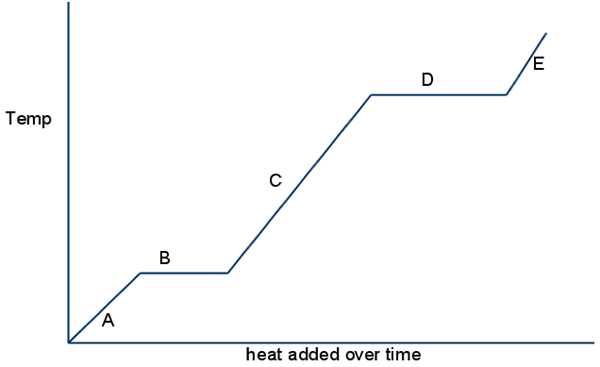
Look at B & D, what happens to kinetic energy?
kinetic energy stays the same
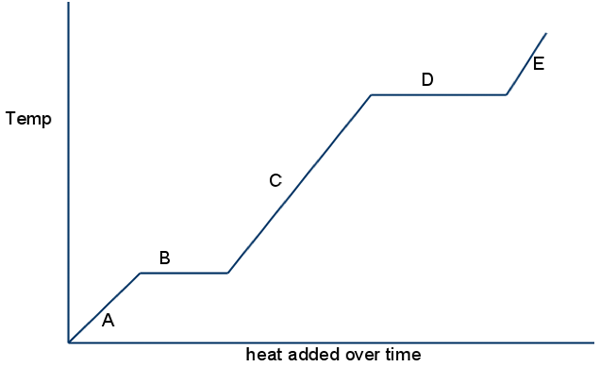
Look at A,C & E, what happens to kinetic energy?
kinetic energy goes up
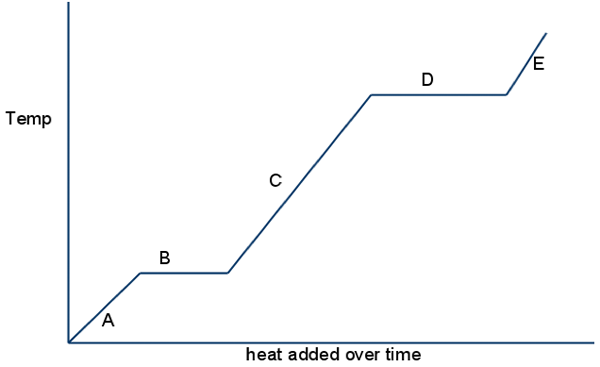
Look at A,C & E, what happens to potential energy?
potential energy stays the same
energy associated with motion
kinetic energy
energy associated with positioning
potential energy