Bohr & Lewis Models, Atoms Extension - Chemistry9
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
21 Terms
What is the area surrounding the nucleus called?
electron shell/cloud
The shell (ring) closest to the nucleus can hold ___ electrons.
2
Shells (rings) 2 & 3 can hold ___ electrons.
8
Shell 4 can hold up to ____ electrons.
18
Why is a atom more reactive when their valence shell isn't full?
This means that the atom is extra reactive. They want to balance out their shell again so they will try to expel an electron (or take one) causing a reaction.
What is a valence shell
The outer most ring of an atom
What is a common fact about all ions? (regarding their valence shell)
They all are "happy" (valence is always full)
Which group on the periodic table has full valence shells always (not ions)
group 18
How many valence electrons would group 16 have?
6 -goes from 1-2 and then it skips the transition metals and jumps back to 13 (so 3-12 do not count) and will be the same number as its category (except when past 13 you only consider 10's place)
How many valence electrons would group 2 have?
2 -goes from 1-2 and then it skips the transition metals and jumps back to 13 (so 3-12 do not count) and will be the same number as its category (except when past 13 you only consider 10's place)
How many valence electrons would group 14 have?
4 -goes from 1-2 and then it skips the transition metals and jumps back to 13 (so 3-12 do not count) and will be the same number as its category (except when past 13 you only consider 10's place)
How many shells would period 5 have?
5 - the number of shells is the number of which period its in (there are seven periods)
How many shells would period 1 have?
1 - the number of shells is the number of which period its in (there are seven periods)
Which order do you draw your atoms in? (direction NESW)
North, South, East, West
When an atom loses/gains electrons it's a __________
ion
What is a positively charged ion called?
cation (loses electrons)
What is a negatively charged ion called?
anion (gained electrons)
How do you draw a Lewis Model?
Write the element symbol and draw ONLY the valence shell electrons around it. if its an ion put the square brackets around it and the charge ex. [N]3-
![<p>Write the element symbol and draw ONLY the valence shell electrons around it. if its an ion put the square brackets around it and the charge ex. [N]3-</p>](https://knowt-user-attachments.s3.amazonaws.com/7c44f554-7d8a-4a9e-99ed-8bcb6aed7c56.jpg)
How do you draw a Bohr model?
draw a nucleus and write your protons number in it. next draw your rings adding your electrons amounts to the appropriate ring (ex. 1st can hold 2) and create pairs of electrons if the amount goes over 4 (ex. look at picture)
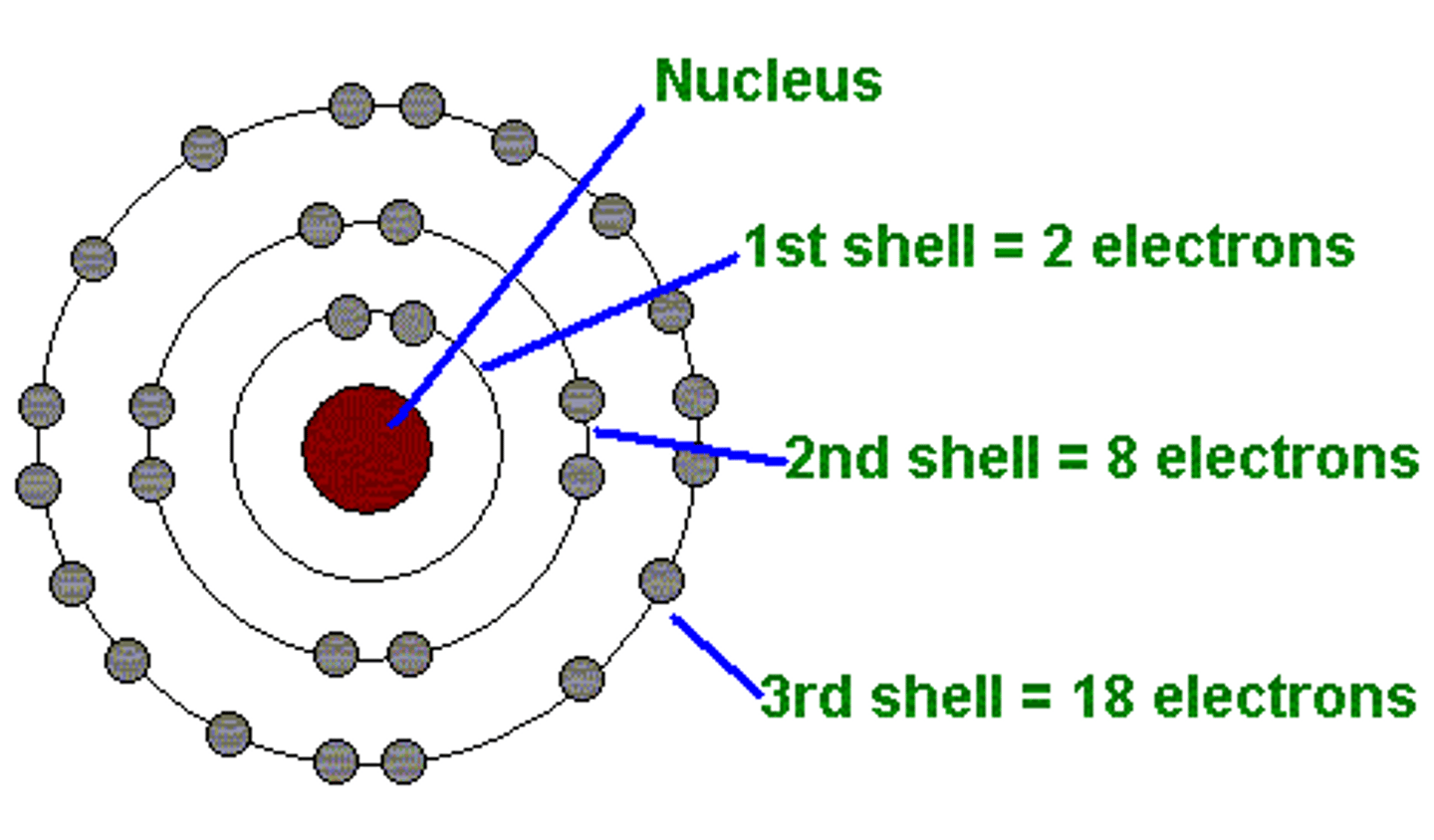
What did Neil Bohr discover?
proposed the orbiting electrons in certain energy levels around the nucleus
Why are the transition metals skipped when looking for their valence shell number in the groups?
They all have varying numbers so the group number theyre in does not apply