Organic Chemistry pKa Values & Conjugate Bases
0.0(0)
Card Sorting
1/19
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
1
New cards
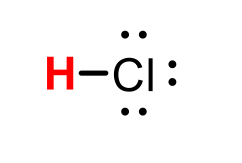
HCl (hydrochloric acid)
pKa = -7
2
New cards
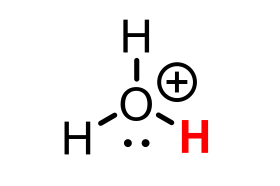
H3O+ (hydronium)
pKa = 0
3
New cards
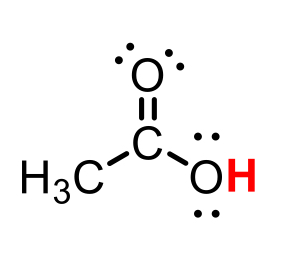
CH3COOH (acetic acid)
pKa = 5
4
New cards
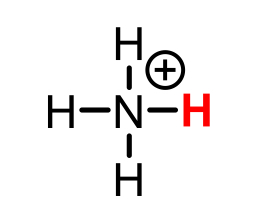
NH4+ (ammonium)
pKa = 9.4
5
New cards
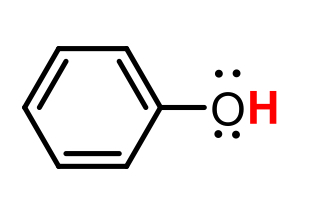
C6H12O (cyclohexanol)
pKa = 10
6
New cards
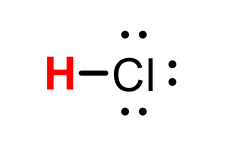
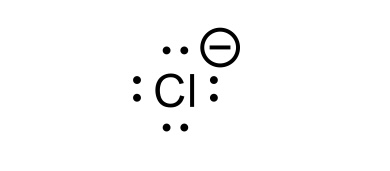
HCl conjugate base
Cl-(chloride)
7
New cards
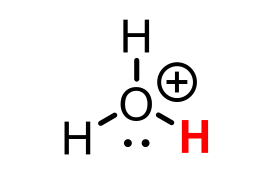
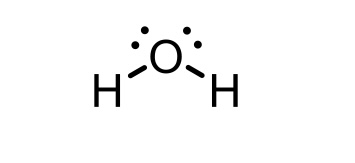
H3O+ conjugate base
H2O (water)
8
New cards
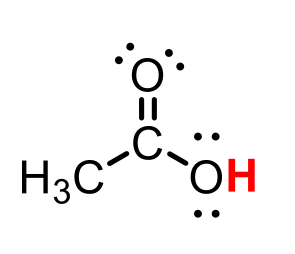
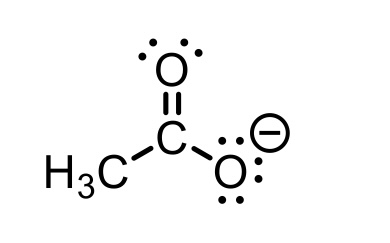
CH3COOH conjugate base
CH3COO- (acetate)
9
New cards
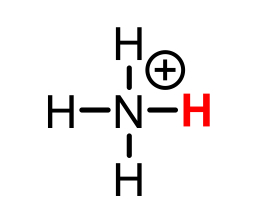
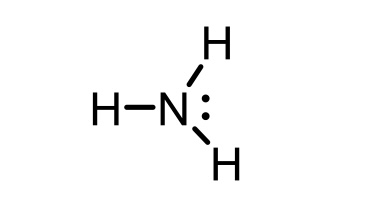
NH4+ conjugate base
NH3 (ammonia)
10
New cards
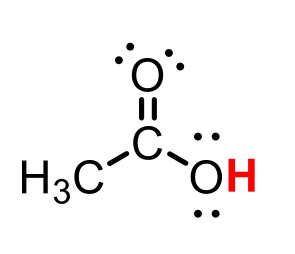
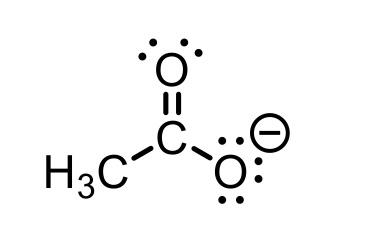
C6H12O conjugate base
C6H11O
11
New cards
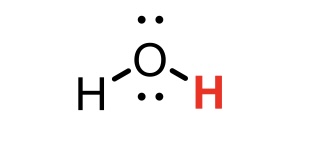
H2O (water)
pKa = 14
12
New cards
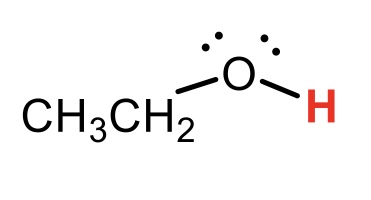
CH3CH2OH (ethanol)
pKa = 17
13
New cards
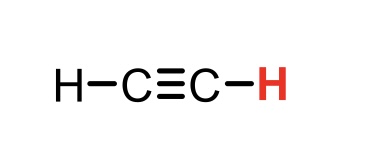
C2H2 (ethyne)
pKa = 26
14
New cards
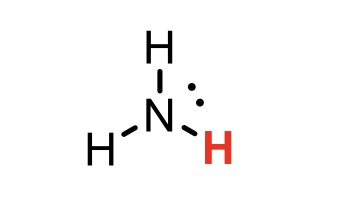
NH3 (ammonia)
pKa = 36
15
New cards
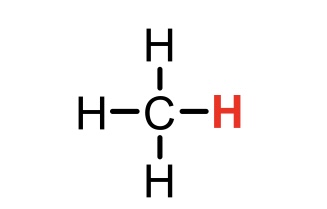
CH4 (methane)
pKa = 60
16
New cards
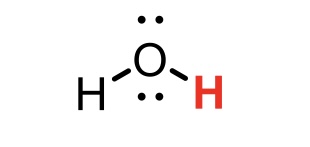
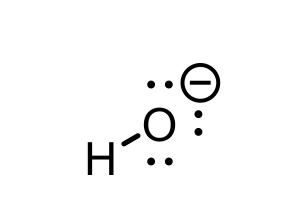
H2O conjugate base
OH-
17
New cards
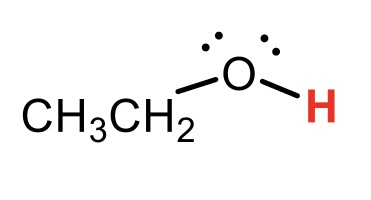
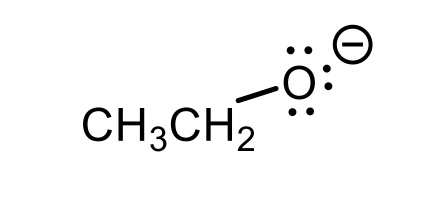
CH3CH2OH conjugate base
CH3CH2O-(ethoxy)
18
New cards
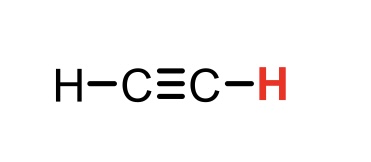
C2H2 conjugate base
C2H-(acetylene)
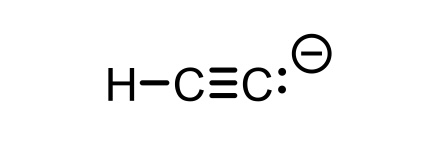
19
New cards
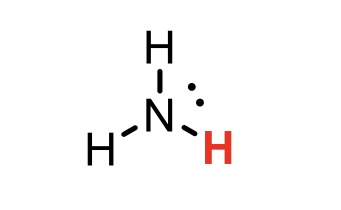
NH3 conjugate base
NH2- (azanide)
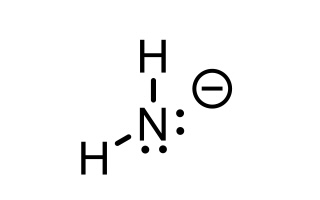
20
New cards
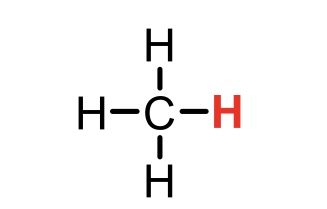
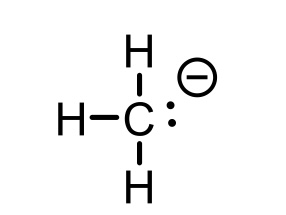
CH4 conjugate base
CH3- (methyl)