class 12 - capturing + using energy 2
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
55 Terms
system
object of study (ex: a cell, body, ecosystem)
note: bio always has open systems- exchange of matter and energy with their surroundings
first law of thermodynamics
law of conservation energy
energy can’t be created nor destroyed
can be transferred
total amount of energy is always the same
second law of thermodynamics
total disorder/entropy of a reacting system increases with every reaction
energy available to do work decreases every time energy changes form (because energy transformations are never 100% efficient)
ex: during a reaction, you lose thermal energy, increasing entropy due to increased random molecular movement
spontaneous
if you left the reactants alone for long enough, you would eventually get the product
non-spontaneous
you can leave the reactants together for an infinite amount of time, but they will not turn into product
Gibbs free energy equation
ΔG = ΔH - TΔS
ΔG = Gibbs free energy = energy available to do work
ΔH = enthalpy
T = temperature (because temperature influences reaction spontaneity)
S = entropy
enthalpy
total amount of energy in the system
which is more likely to be spontaneous: -ΔH or +ΔH?
-ΔH is more likely to be spontaneous as it indicates a release of energy from the system, favoring reaction spontaneity.
-ΔH = reactants have more potential energy than products, releases energy
+ΔH = products have more potential energy than reactants, gains energy
-ΔH is more likely to be spontaneous as a release of energy is favored, as the system would be moving toward a lower-energy, more stable state
entropy
measure of disorder or randomness in a system
which is more likely to be spontaneous: -ΔS or +ΔS?
-ΔS = reactants are more disordered
+ΔS = products are more disordered
+ΔS is more likely to be spontaneous, as system will favor becoming more disordered
+ΔG: is it ender/exergonic? is it spontaneous or not spontaneous? what would the graph look like?
+ΔG = endergonic, not spontanous
products have more free energy than reactants
not spontaneous as reaction requires a sustained input of energy
think about it as you can’t go up a hill without adding energy
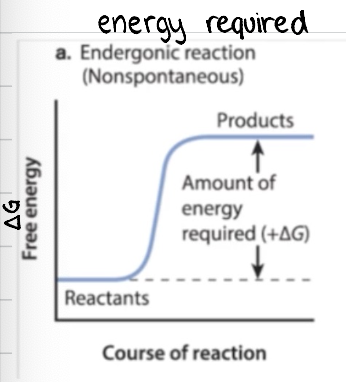
-ΔG: is it ender/exergonic? is it spontaneous or not spontaneous? what would the graph look like?
-ΔG = exergonic, spontaneous
reactants have more free energy than products
reaction releases energy (think of exo = exit)
relationship between spontaneity and speed of reaction
spontaneous does not mean fast reaction! they are unrelated.
what would happen if you didn’t have enough ATP for an endergonic reaction?
the reaction wouldn’t get to the final product, because there isn’t enough accessible energy to power the reaction
what would happen if you didn’t have enough ATP for an exergonic reaction?
the reaction could still proceed, but it may not be as efficient or may occur at a slower rate
-ΔH
negative enthalpy change
reactants → products release energy
reactants have more potential energy, products have less potential energy
favors spontaneity
+ΔH
positive enthalpy change
products have more potential energy
reactants → products gains energy
does not favor spontaneity
-ΔS
negative entropy change
reactants are more disordered
reactants → products, system becomes more ordered
does not favor spontaneity
+ΔS
positive entropy change
products are more disordered
reactants → products, system becomes more disordered
favors spontaneity
-ΔH, -ΔS
spontaneous at low temperatures
non spontaneous at high temperatures
enthalpy and entropy changes
+ΔH, -ΔS
nonspontaneous at all temperatures
-ΔH, +ΔS
spontaneous at all temperatures
+ΔH, +ΔS
spontaneous at high temperatures
nonspontaneous at low temperatures
exergonic
requires energy to form bonds (ex: dehydration synthesis), proceeds spontaneously (-ΔG)
endergonic
releases energy when breaking bonds (ex: ATP hydrolysis), not spontaneous (+ΔG)
energetic coupling
the driving of a non spontaneous reaction (-ΔG) by a spontaneous reaction (+ΔG)
pairs ATP hydrolysis (spontaneous exergonic) with nonspontaneous endergonic reaction
coupled reaction
a +ΔG reaction (endergonic) is paired with a -ΔG reaction (exergonic). If the total ΔG is negative (exergonic, spontaneous), then the coupled reaction will occur together spontaneously
ATP hydrolysis
ATP + H2O → ADP + Pi + energy
goes from less stable ATP to more stable ADP
exergonic process
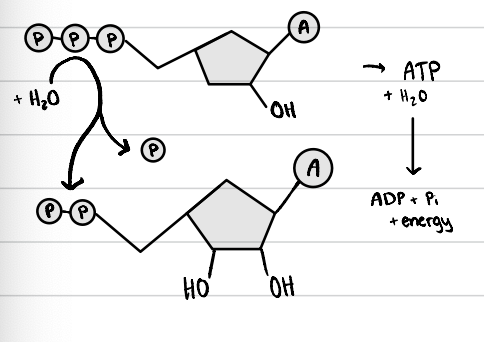
enzyme
protein based, made of amino acids
acts as a catalyst to accelerate the rate of a chemical reaction by lowering the activation energy (EA) of a reacting system and stabilizing the transition state of reactants
activation energy (EA)
energy input necessary to reach transition state
transition state
the hump that reactants have to get over to make the product
the brief time in a chemical reaction where the reactant’s bonds are broken and product’s bonds are formed
when are enzymes good?
enzymes are useful because they are able to speed up reactions under mild conditions, so you don’t have to add heat and accidentally denature a molecule.
additionally, they don’t get consumed in the process
substrate
the reactant in an enzyme-catalyzed reaction
process of a substrate being catalyzed by an enzyme
enzyme is separate from substrate
enzyme-substrate complex - substrate binds to enzyme
enzyme-product complex - enzyme sonverts substrate
product is released, enzyme remains unchanged
catabolism
breaks down molecules into smaller units
released energy is used to product ATP from ADP and Pi
anabolism
builds molecules from smaller units
requires energy input
chemical equilibrium
rate of forward reaction = rate of reverse reaction
the concentration of reactants and products don’t change
describe how enzyme structure is related to function
enzyme’s tertiary structure determines shape of active site
altering tertiary structure (ex: by pH, heat, denaturation) → active site may change shape, so substrate may no longer be able to bind properly, blocking reaction
enzyme’s relationship with spontaneity
enzyme’s don’t change the ΔG of the reaction
cofactor
enzymes don’t always work alone, they sometimes use a cofactor
cofactor- non-protein, non substrate molecule helps enzyme activity
active site
location on enzyme where substrate binds
allosteric site
the place on an enzyme that a molecule (not a substrate) binds to inhibit or stimulate enzyme activity
competitive inhibition
inhibitor binds to active site
blocks substrate from binding
direct competition with substrate

non-competitive inhibition
inhibitor binds elsewhere on enzyme (not active site), such as allosteric site
changes enzyme shape → prevents substrate binding or conversion
substrate may still bind, but cannot be converted into product
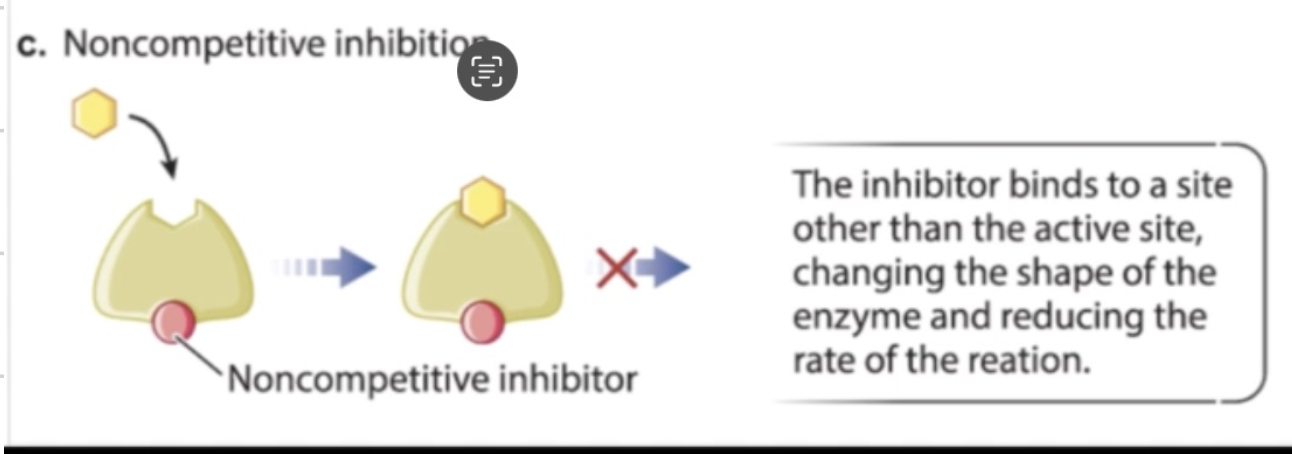
what factors speeds up a reaction?
increasing presence of enzyme activity/number of enzymes
lower EA
increasing temperature to a certain point (avoiding denaturing)
increasing substrate concentration until Vmax
adding cofactors
what factors slows down a reaction?
decreasing heat = decreased rate
extreme temperatures (too high temperature → denaturation)
competitive / non-competitive inhibition (reversible vs irreversible)
pH out of enzymes range
mutation (impacts primary structure)
impact of enzyme denaturation
changing enzyme structure changes enzyme function - enzyme may no longer bind
Michaelis-Menten plot
shows enzyme kinetics
as substrate increases, reaction rate reaches Vmax (maximum velocity)
on a Michaelis-Menten plot, how do you increase the rate beyond Vmax?
you must increase the enzyme concentration
Vmax
maximum reaction rate (all active sites developed) possible with given amount of enzymes
you know when you are at Vmax because the line flattens out
Km
substrate concentration at ½ Vmax
indicator of enzyme-substrate affinity
what does a low Km mean?
the substrate saturates quickly, since a low Km means that the enzyme reaches half of its Vmax at a lower substrate concentration
Therefore, it doesn't need much substrate to saturate, indicating high enzyme and substrate affinity
what does a high Km mean?
the substrate doesn't saturate quickly, indicating that a higher concentration of substrate is required to reach half of its Vmax
This suggests a lower affinity between enzyme and substrate, and that it would take longer to saturate
what does competitive inhibition do on a Michaelis Menten plot:
competitive inihibition - increases Km (needs more substrate), Vmax changed
Km is increased because you can’t outcompete a competitive inhibitor
what does noncompetitive inhibition do on a Michaelis Menten plot:
decreases Vmax (less overall activity), Km unchanged
Km is unchanged because substrate can still bind, but inhibitor prevents reaction from flowing