Distillation
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
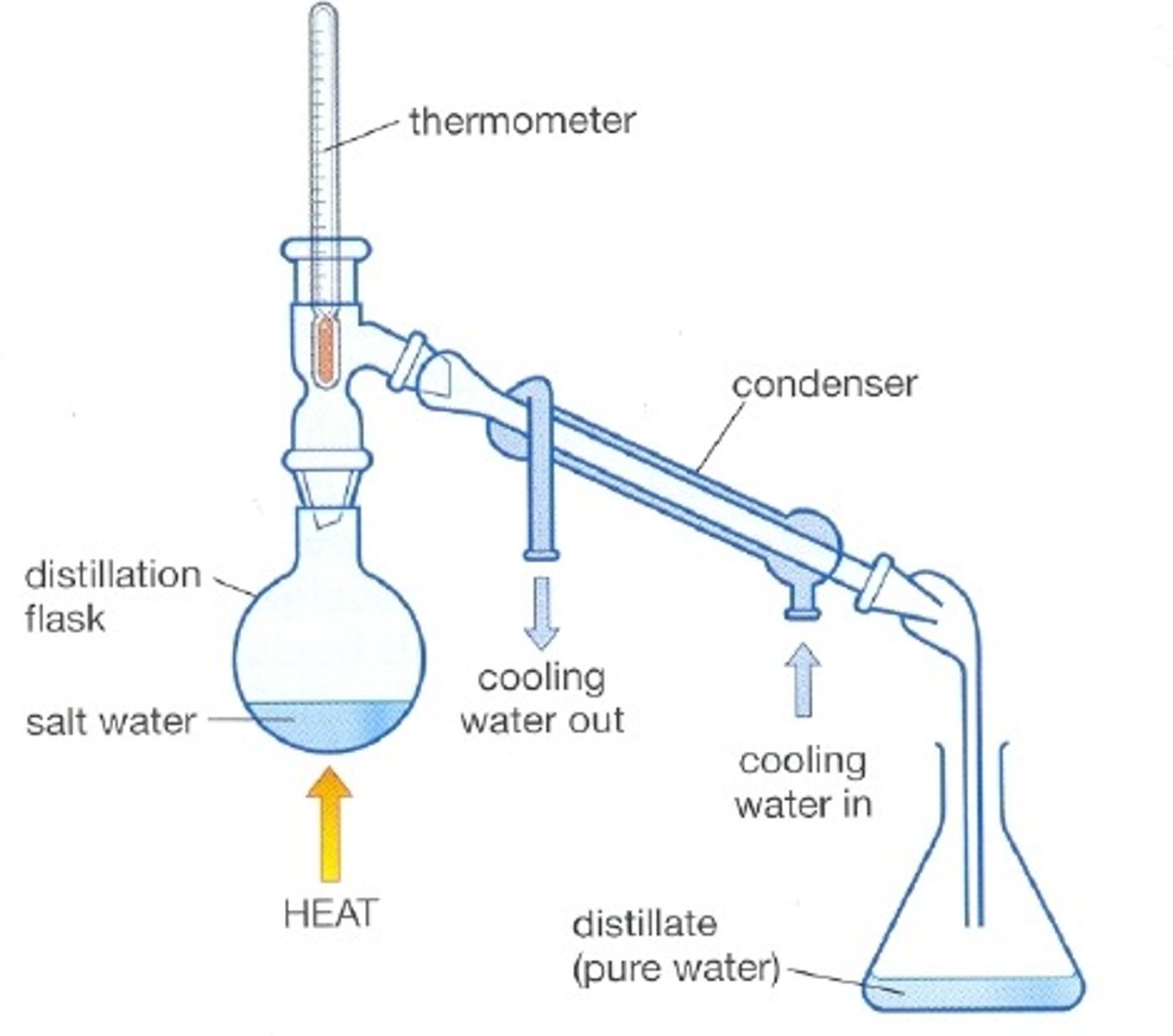
Distillation
A process that separates the substances in a solution based on their boiling points
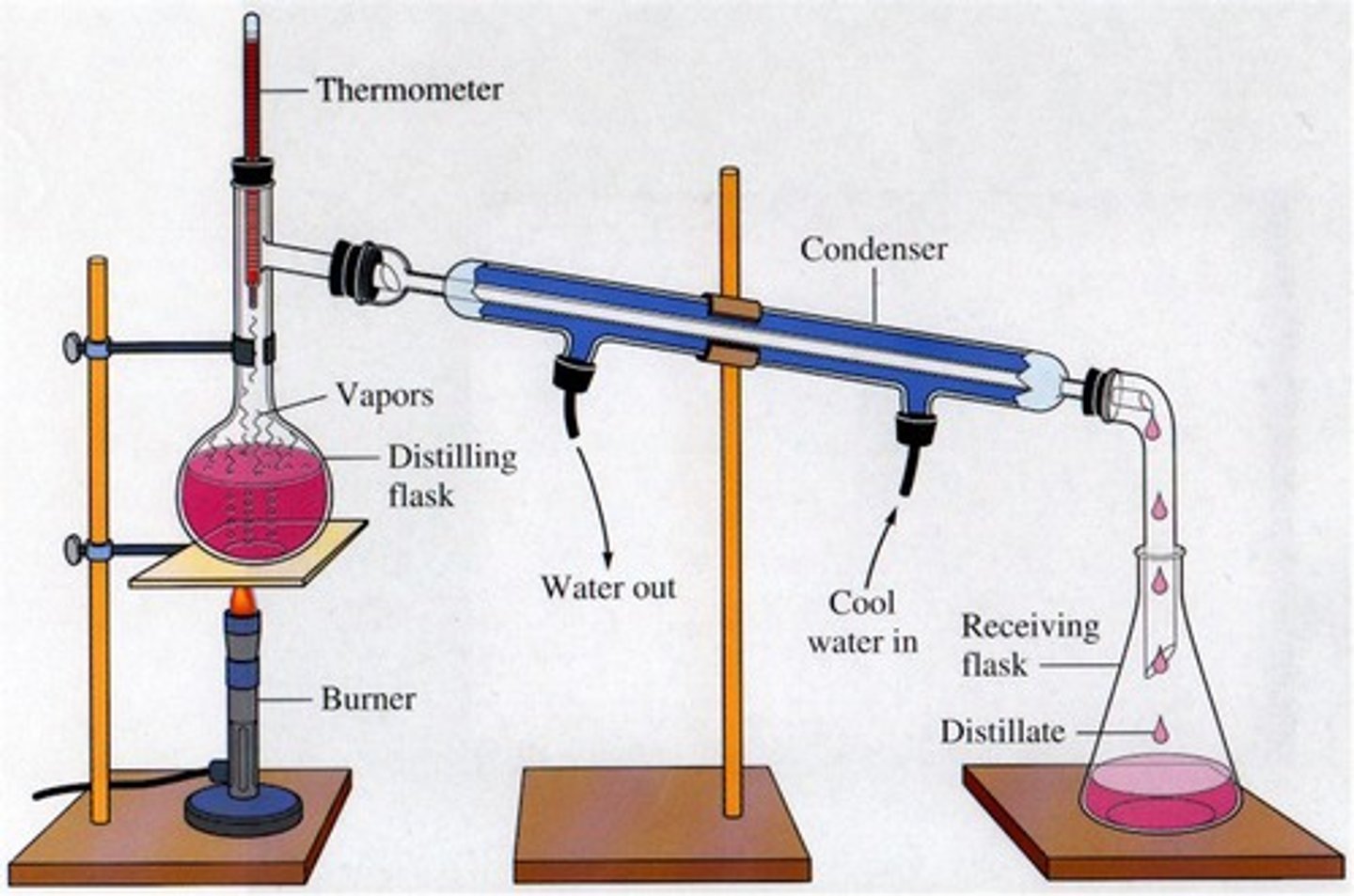
the process of distillation
evaporation and condensation cycling
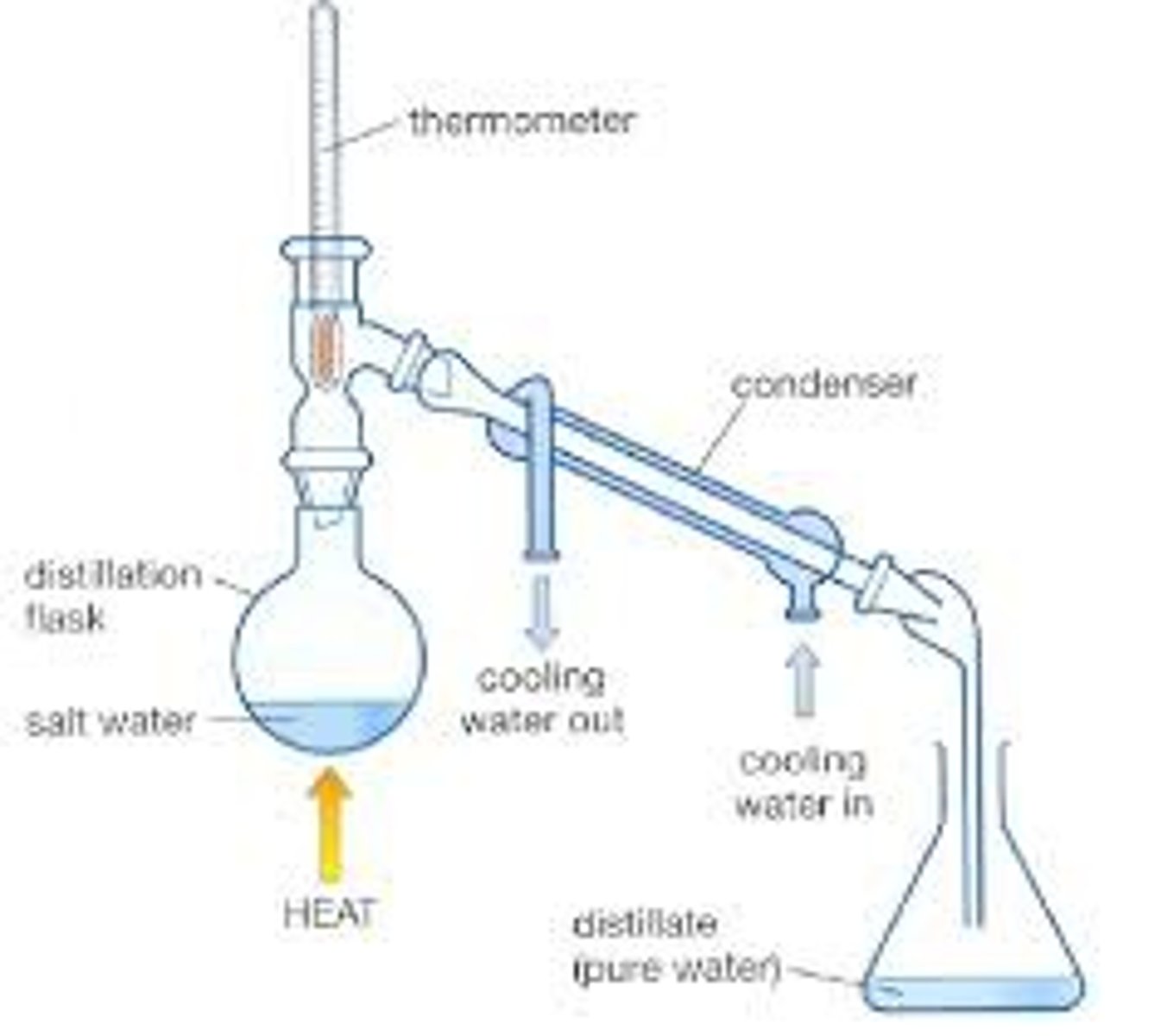
the types of distillation
simple, steam, fractional, and column
when should simple distillation be used
can be used if the states of matter are different and the boiling point difference between liquids is 70 degrees or more (25-100 degree difference)
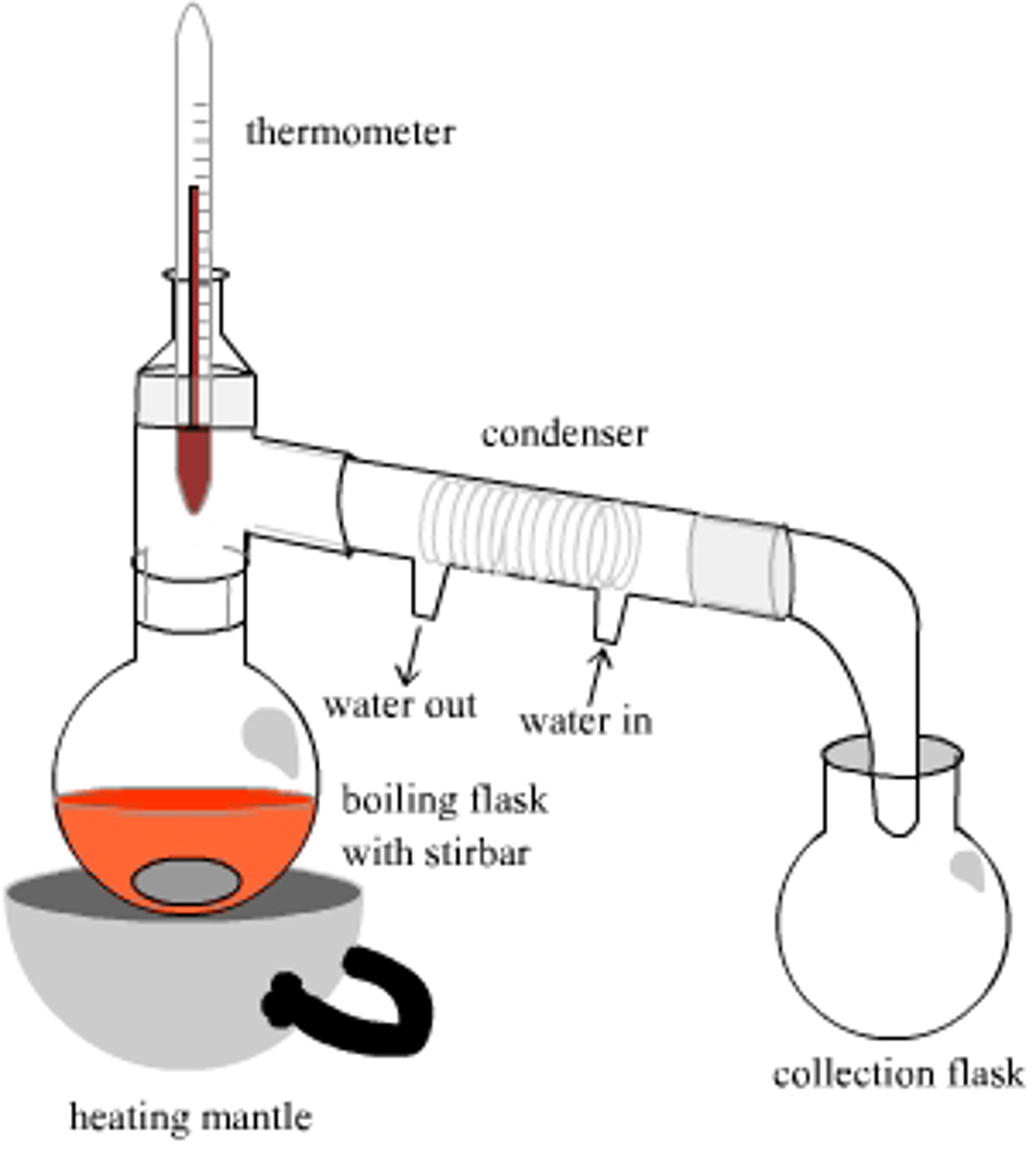
when should fractional distillation be used
when the compounds' boiling point difference is less than 25 degrees
when should steam distillation be used
similar to simple distillation, but steam is used in the distilling flask
components of crude oil
Toluene, xylene, hydrocarbons
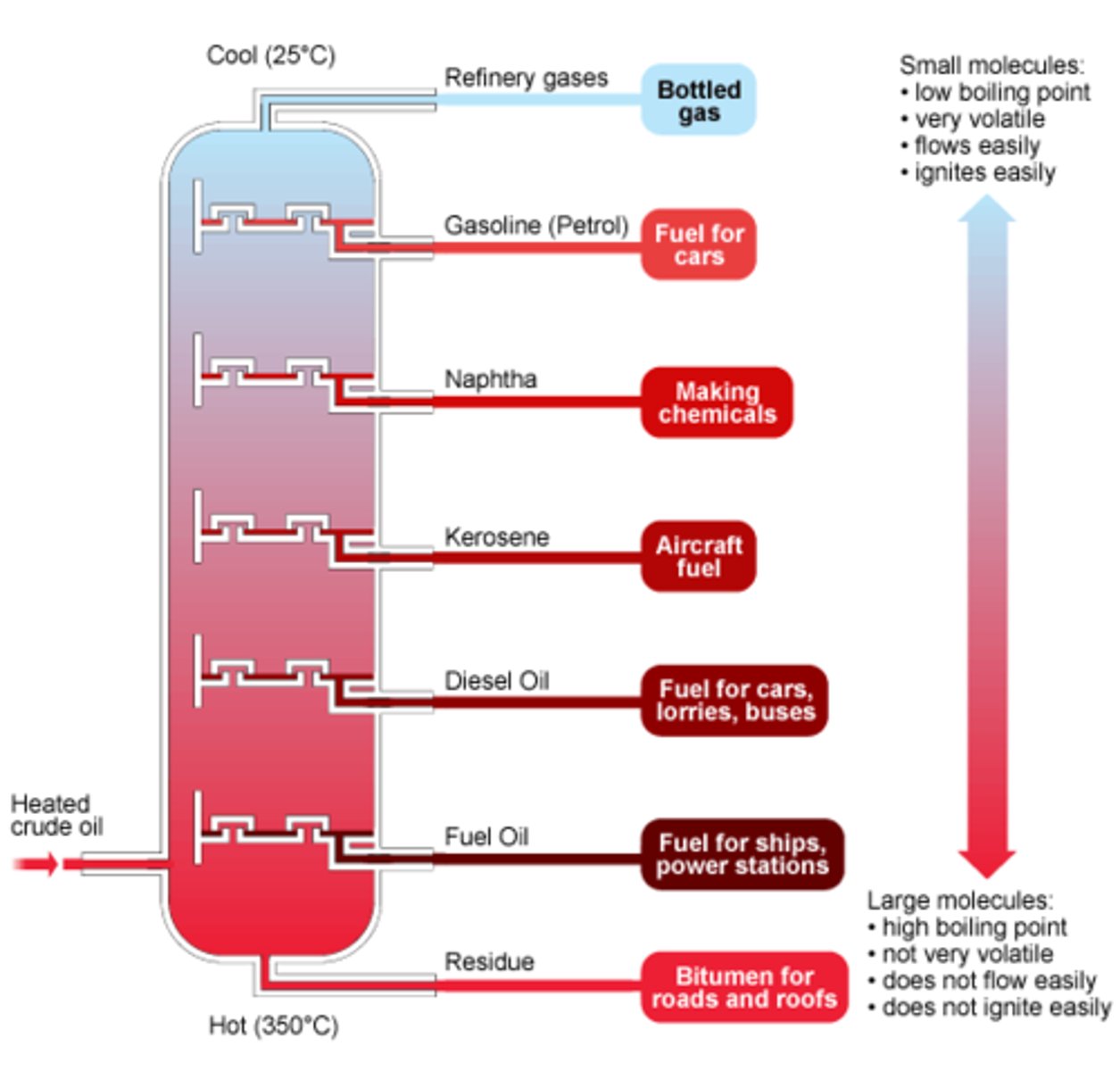
WHERE is fractional distillation COMMONLY used
in oil manufacturing
types of column distillation
packed and unpacked
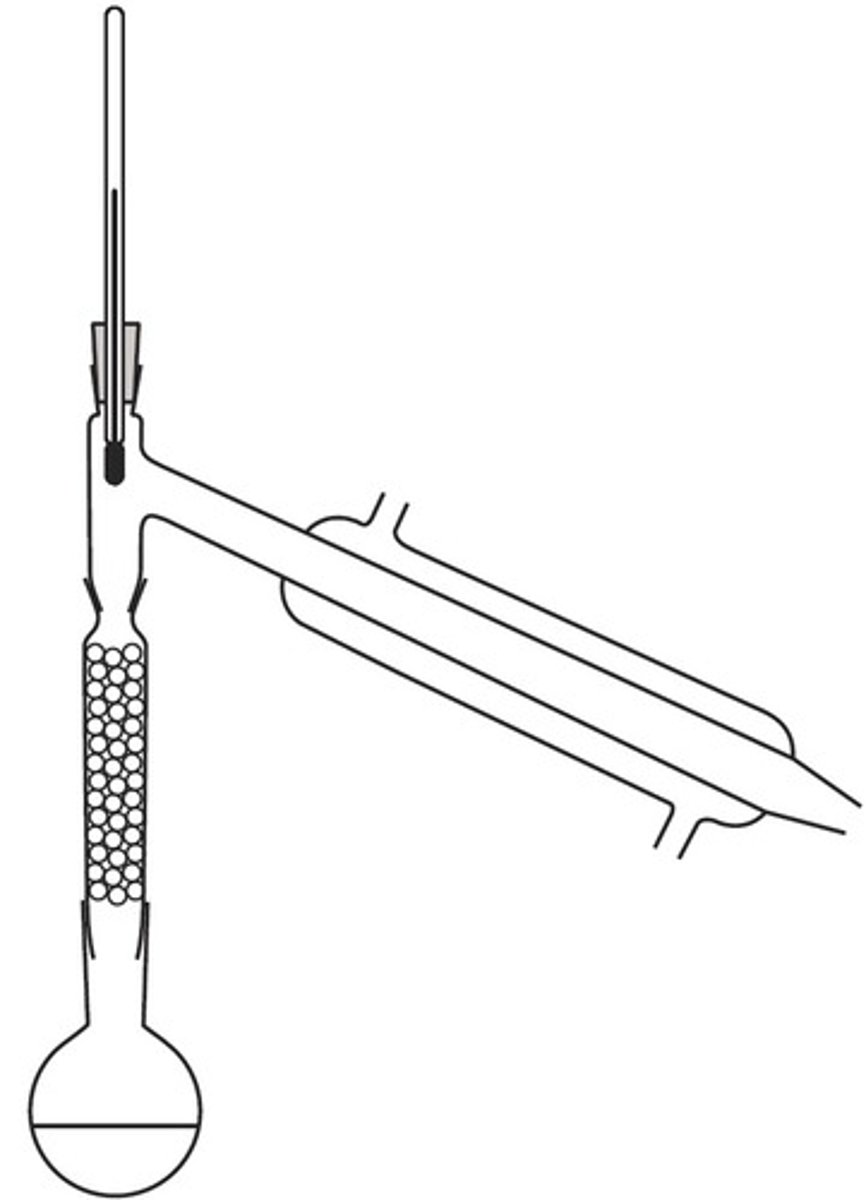
vacuum distillation
- should be used if boiling points are over 150 degrees C to prevent degradation of the product
packed column distillation does what
lower the energy of the molecule coming in

efficiency of distillation
packed column, empty column, and simple
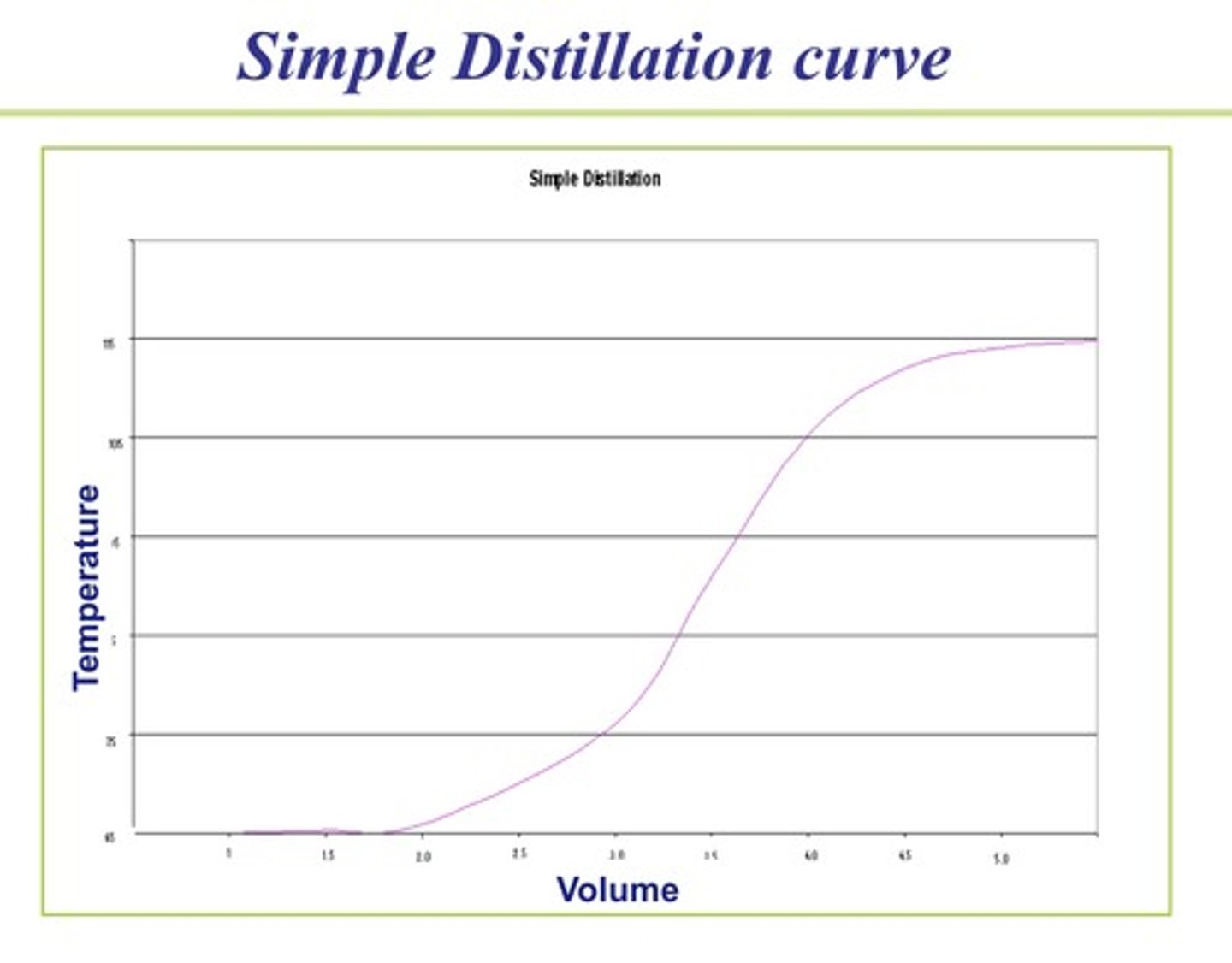
what factors affect the efficiency of distillation
the types of distillation set up
mixture type
the boiling point difference
the surface area of the boling point flask
pressure
rate of heating
theoretical plate
represents one vaporization/condensation cycle
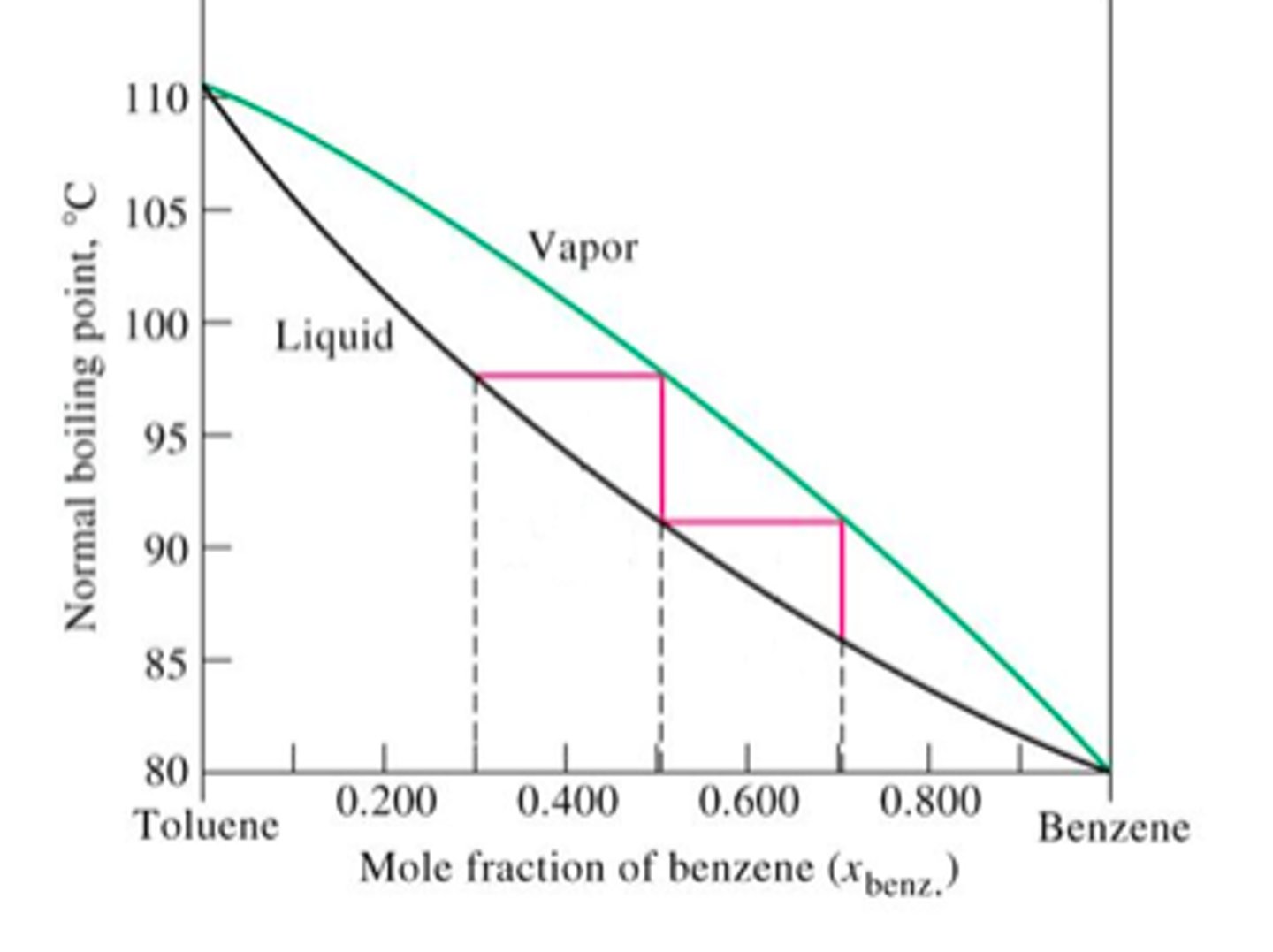
Which compound has a higher boiling point: acetone or toluene
toluene
toluene
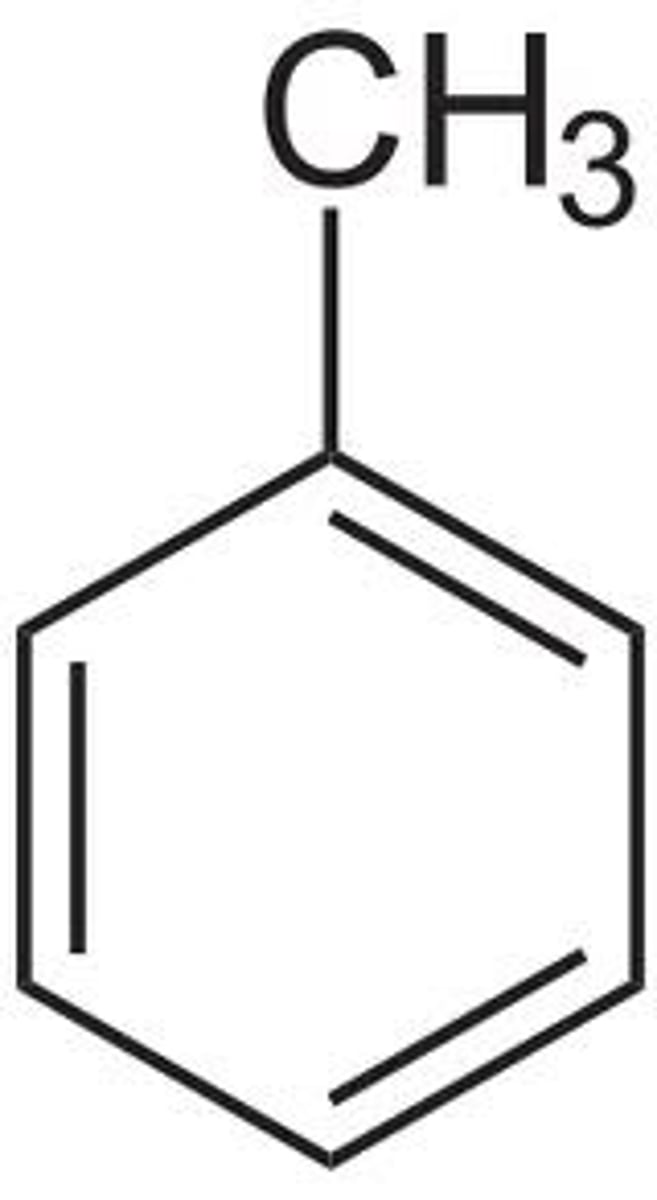
benzene with CH3
acetone boiling point
56°C
Toluene boiling point
111
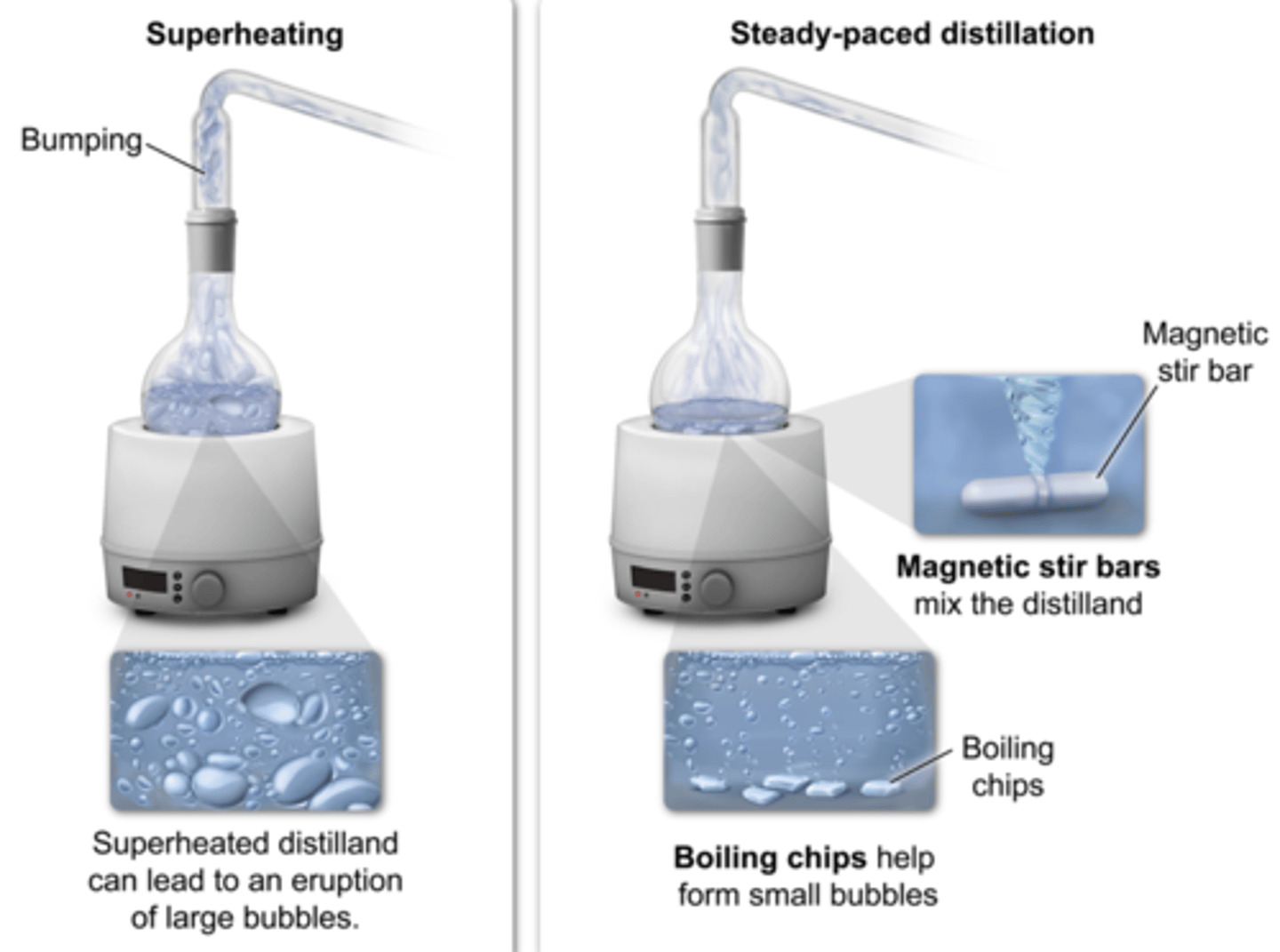
what is the purpose of boiling chips
ensure smooth boiling
gas laws related to distillation
Charles Law and Gay-lussac law
charles law
with constant pressure, temperature is proportional to volume
gay-lussac law
at constant volume, pressure is proportional to temperature.
volatility
low BP and high vapor pressure
Pressure relationship to boiling point
directly proportional