C5 - Energy Changes
1/20
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
What is an exothermic reaction?
A reaction that transfers energy to the surroundings
What happens to the temperature of the surroundings in an exothermic reaction?
The temperature increases
What are some examples of exothermic reactions?
Combustion, neutralisation, and Oxidation reactions
What is an endothermic reaction?
A reaction that takes in energy from the surroundings
What happens to the temperature of the surroundings in an endothermic reaction?
It decreases
What are some examples of endothermic reactions?
Thermal decomposition, photosynthesis
What type of reaction happens in a self- heating can or hand warmer?
Exothermic reaction
What type of reaction happens in a sports injury cold pack?
Endothermic
Temperature Change Practical
Use a measuring cylinder to measure 30 cm³ of dilute hydrochloric acid
Pour the acid into the cup and stand the polystyrene cup into a beaker
Use a thermometer to measure the temperature of the acid and record it on a table
Use measuring cylinder to measure 5 cm³ of sodium hydroxide solution and pour into the cup
Place plastic lid on the cup, put thermometer through the hole in the lid, and gently stir the solution
Continuation of practical
When the reading stops changing, record the highest temperature reached
Rinse out and dry the polystyrene cup
Repeat the whole experiment using 10 cm³ of sodium hydroxide solution
Carry out the experiment several times, increasing the volume of sodium hydroxide solution by 5 cm³ until you reach 40 cm³.
Repeat the whole experiment to get two sets of results. Use these to calculate a mean value for the maximum temperature reached for each volume, and then plot a results graph
What does a reaction profile show?
The energy changes during a chemical reaction
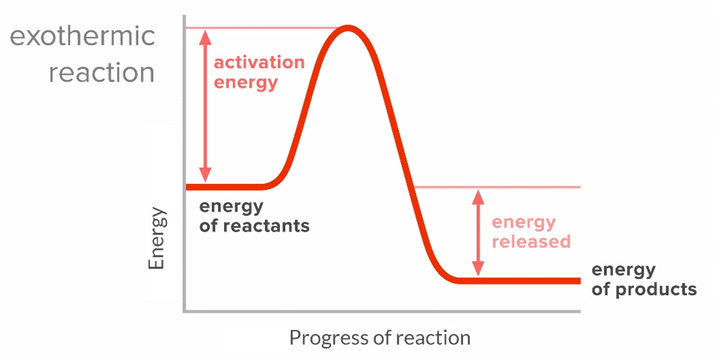
What has more energy in an exothermic reaction profile?
Reactants have more energy than products
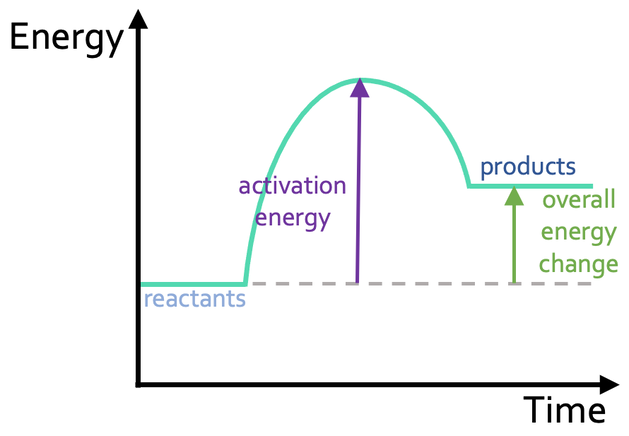
What has more energy in an endothermic reaction profile?
Products have more energy than reactants
What is the activation energy?
The minimum amount of energy the reactants need to collide with each other and react
Exothermic Reaction Energy Profile
Endothermic Reaction Energy Profile
What happens to bonds during chemical reactions?
Bonds in reactants are broken
New bonds are made in products
What happens to the energy when bonds are broken?
Energy is taken in (endothermic)
What happens to the energy when bonds are made?
Energy is released (exothermic)
What units are bond energies measured in?
kJ/mol
What formula can be used to estimate energy change?
Energy change = Energy in (bonds broken) - energy out (bonds made)