Excitable tissue Nerves; Synapses; and Neuromuscular Junctions
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
What is the function of orthograde axonal transport?
To move cellular components away from the cell body (down the axon) toward the axon terminals
What determines the frequency of action potentials on target neurons?
The combined effect of excitatory post-synaptic potentials (EPSPs) and inhibitory post-synaptic potentials (IPSPs) and pre- and postsynaptic inhibition
What are the four main parts of a single neuron?
Input zone(dendrites and cell body); axon hillock or trigger zone; axon or conducting zone; axon terminal or output zone
What is the primary function of dendrites in the neuron?
They are numerous extensions housed in the cell body that increase the surface area available for receiving signals from other neurons
What structures constitute the Input Zone of a neuron?
The cell body and dendrites
What is the function of the input zone?
It receives and integrates incoming chemical signals from other neurons that bind to protein receptors (ligand-gated ion channels)
Approximately how many dendrites can some neurons have?
Up to 400;000 dendrites
What is the definition of an axon (nerve fiber)?
A single; elongated; tubular extension that conducts action potentials away from the cell body (orthograde) and eventually terminates at other neurons; muscle cells; and endocrine cells
How long can axons vary in size?
From less than 1 mm to longer than a meter in neurons that communicate with distant parts of the nervous system or peripheral muscle/organs
What is the axon hillock or initial segment?
The first portion of the axon plus the region of the cell body from which the axon leaves
Why is the axon hillock considered the neuron's trigger zone?
It is the site where action potentials are triggered or initiated from the summation of all the incoming signals from other neurons
How are action potentials conducted to the output zone?
They are conducted along the axon from the axon hillock (orthograde) to the highly branched ending at the axon terminals that release chemical messengers
Functionally; what regions constitute the output zone?
The axon terminals
What is Wallerian degeneration?
The degeneration of the part of the axon distal to the cut if the axon is severed; which occurs because axon transport ceases; proving the neuron's cell body maintains the functional and anatomic integrity of the axon
What direction is orthograde transport; and what motor protein is required?
Transport towards the axon terminals along microtubules; requires the molecular motor protein kinesin
What are the speeds associated with fast and slow orthograde transport?
Fast is approximately 400 mm/day; slow is approximately 1 mm/day
What direction is retrograde transport; and what motor protein is required?
Transport from the axon terminals to the cell body; requires the molecular motor protein dynein
What is the speed of retrograde transport?
Approximately 200 mm/day
What is the approximate membrane potential of all cells at rest?
Approximately -70mV
What is the definition of resting membrane potential?
The constant membrane potential in the cells of nonexcitable tissues and those of excitable tissues when they are at rest (not producing electrical signals)
What tissues are classified as excitable tissues; and what ability do they possess?
Nerve cells and muscle cells; they have the ability to produce rapid; transient changes in their membrane potential when excited that serve as electrical signals
What causes the inside of the cell to become more negative relative to the outside at rest?
The action of the Na+-K+ ATPase; moving 3 Na+ out and only 2 K+ in; resulting in an excess of positive charges in the Extracellular Fluid (ECF) and negative charges in the Intracellular Fluid (ICF)
How are changes in membrane potential achieved?
Changes in ion movement are brought about by changes in membrane permeability via the opening and closing of ion channels in response to triggering events that alter ion flow across the membrane
What equation is used to determine the membrane potential (voltage) based on ion permeabilities?
The Goldman-Hodgkin-Katz Equation (Vm
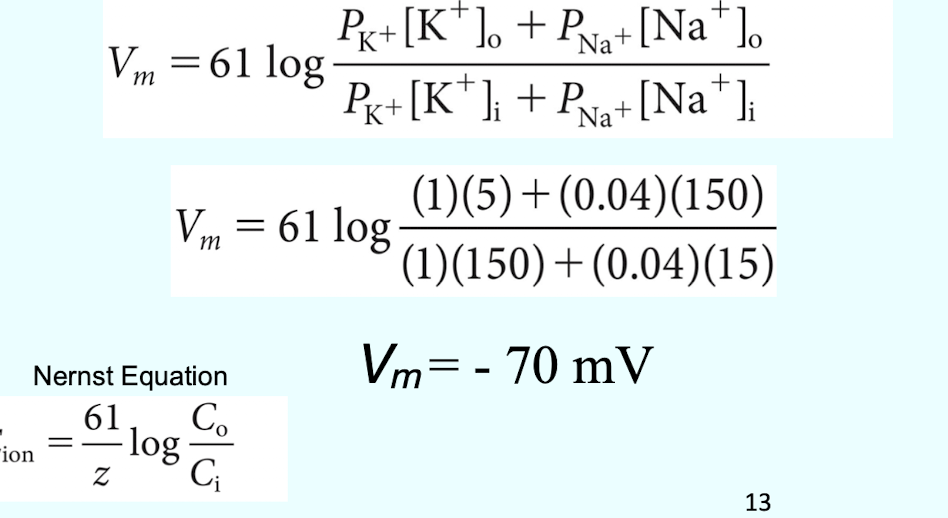
What is the Nernst Equation used to calculate?
The equilibrium potential (Eion) for a single ion
VIGNETTE: During the rising phase of an action potential; the permeability of Na+ (PNa) increases drastically (e.g.; from 0.04 to 1000). According to the Goldman-Hodgkin-Katz equation; what potential does the membrane approach?
Vm = +61mV; the membrane potential approaches the Equilibrium Potential of Na+ (ENa)
VIGNETTE: If the permeability of K+ (PK) increased significantly (e.g.; from 1 to 1000); what would the resulting membrane potential approach?
Vm = -90mV; the membrane potential approaches the Equilibrium Potential of K+ (EK)
What are leak channels?
Channels which are open all the time and permit unregulated leakage of a specific ion across the membrane
What are gated channels?
Channels that can be opened or closed; permitting ion passage through the channels when open and preventing ion passage when closed; gate opening and closing occurs in response to a triggering event
List the four kinds of gated channels.
Voltage gated channels (respond to changes in membrane potential);
Chemically gated channels (respond to binding of a specific extracellular chemical messenger);
Mechanically gated channels (respond to stretching or other mechanical deformation);
Thermally gated channels (respond to local changes in temperature)
What are the two primary forms of electrical signals in excitable tissue?
Graded or electrotonic potentials (local; nonpropagated; short-distance signals);
Action potentials (propagated; long-distance signals)
Name the types of potentials categorized as graded potentials.
Synaptic potential (EPSP or IPSP);
generator or receptor potential;
pacemaker potentials;
slow-wave potentials;
end-plate potentials (EPP) at the neuromuscular junction
What is a graded potential?
Local changes in membrane potential that occur in varying grades or degrees of magnitude or strength
How are graded potentials usually produced?
By a specific triggering event that causes gated ion channels to open; resulting in ion movement; most commonly depolarizing due to net Na+ entry
Why are graded potentials considered "local"?
They are confined to a small; specialized region of the total plasma membrane near the open ion channel and do not propagate down the membrane
What are the two important characteristics relating a triggering event to a graded potential?
1) The stronger the triggering event; the larger the amplitude of the resultant graded potential;
2) The longer the duration of the triggering event; the longer the duration of the graded potential
What is the temporarily depolarized region where a graded potential occurs locally called?
The active area
How does the magnitude of a graded potential change as it moves from the initial active area?
It progressively diminishes; meaning the spread of a graded potential is decremental (gradually decreases)
VIGNETTE: When a neuron is stimulated by subthreshold intensities (e.g.; 0.2 to 0.8 times threshold intensity); what kind of response is observed on the membrane potential graph?
A local response (a graded potential) that does not reach the firing level (-55mV) to initiate a propagated action potential
Name the five specific examples of graded potentials.
Post-synaptic potentials (EPSP and IPSP) synapses between neurons;
receptor or generator potentials;
end-plate potentials (EPP) neuromuscular junction;
pacemaker potentials;
slow-wave potentials
Describe the key features of an action potential (AP).
They are rapid; large (100mV) changes in membrane potential; they are conducted or propagated nondecrementally (do not diminish in strength) throughout the entire membrane; they serve as faithful long-distance signals
What event marks the onset of the absolute refractory period during an action potential?
Closure of the Na+ inactivation gate
What process occurs at the threshold potential (Step 2 in the action potential diagram)?
Na+ voltage-gated channels open and PNa+ rises
What causes the explosive depolarization (rising phase) of the action potential (Step 3)?
Na+ enters the cell; causing explosive depolarization to +30 mV
What two main events occur at the peak of the action potential (+30 mV) (Step 4)?
Na+ inactivation gate closes and PNa+ falls; resulting in net movement of Na+ into the cell ceasing; simultaneously K+ activation gate opens and PK+ rises
What ion movement causes repolarization (falling phase) of the action potential (Step 5)?
K+ leaves the cell (K+ efflux) causing repolarization to resting potential
What causes hyperpolarization (after-hyperpolarization) (Step 7)?
Further outward movement of K+ through still-open K+ channels; making the membrane briefly more negative
What characterizes the rising phase of the action potential (threshold to +30 mV)?
Na+ influx (Na+ entering the cell) induced by an explosive increase in voltage Na+ Channel opening/permeability at the threshold
Why does hyperpolarization occur?
The voltage-gated K+ channels are slow to close; resulting in persistent increased permeability to K+; causing the interior of the cell to become transiently even more negative than resting potential
Once an action potential is initiated at the axon hillock; what further trigger events are required to conduct the impulse?
None; the impulse is conducted throughout the neuron without further stimulation
What are the two types of conduction that action potential propagation occurs via?
Contiguous conduction and saltatory conduction
Describe contiguous conduction.
It involves spreading the action potential along every membrane patch down the axon's length
Describe the all-or-none law.
Excitable membrane either responds to a triggering event with a maximal action potential that spreads nondecrementally throughout the membrane; or does not respond with an action potential at all
In contiguous conduction; how does the local current flow spread depolarization to the adjacent inactive area?
Local current flows from the active area (at the peak of the AP) to the adjacent inactive area; depolarizing it from resting potential to threshold potentia
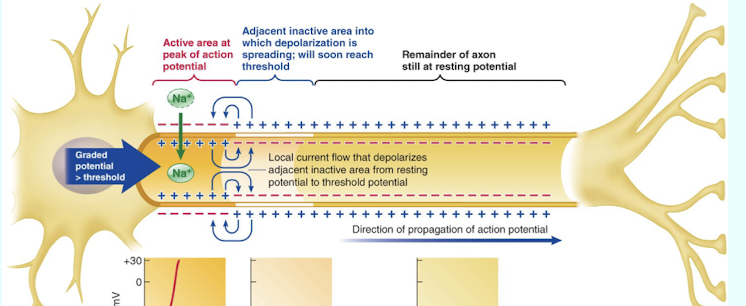
What is the absolute refractory period (ARP)?
The period during the rising phase and peak of the action potential (where Na+ channels are inactive) when the cell will not elicit another action potential if stimulated
What phases of the AP correspond to the relative refractory period?
The late falling phase and hyperpolarization (where K+ channels are open)
How does the nervous system distinguish between two stimuli of varying strengths (e.g.; warm vs. hot) if both generate action potentials of the same magnitude?
The answer lies partly in the frequency with which the action potentials are generated; a stronger stimulus triggers a greater number of action potentials per second; but NOT a larger action potential
What two factors determine the velocity (speed) of action potential conduction down an axon?
Whether the nerve fiber is myelinated; and the nerve fiber diameter (increased diameter increases speed)
In what type of fiber does contiguous conduction occur?
Unmyelinated fibers
What type of conduction is the faster method of propagation; and in what fibers does it take place?
Saltatory conduction; takes place in myelinated fibers
What structures are found concentrated at the Nodes of Ranvier in a myelinated fiber?
Voltage-gated Na+ and K+ channels
How does saltatory conduction propagate the action potential?
Local current flow depolarizes an adjacent inactive node from resting potential to threshold; causing the action potential to jump from node to node
VIGNETTE: A 30-year-old patient presents with new onset vision changes and increasing fatigue. Workup reveals an autoimmune condition where the body attacks the myelin sheath in the CNS. What pathophysiologic condition is described?
Multiple Sclerosis (MS); a condition in which nerve fibers lose their myelin; resulting from the body's defense system erroneously attacking the myelin sheath surrounding myelinated nerve fibers
What is the typical age range for the onset of Multiple Sclerosis (MS)?
Between the ages of 20 and 40
What is the relationship between nerve fiber diameter and conduction velocity?
As fiber diameter increases; conduction velocity increases
What are the three structures a neuron may terminate on?
A muscle (causing contraction); a gland (causing secretion); or another neuron (conveying an electrical message along a nerve pathway)
What is the junction between two neurons called?
A synapse
What is the junction between a neuron and a muscle cell called?
A neuromuscular junction
What are the two types of synapses?
Electrical and chemical
Describe the characteristics of electrical synapses.
Two neurons connected by gap junctions; allowing charge-carrying ions to flow directly between cells in either direction (bidirectional); resulting in unbroken; extremely rapid; unregulated transmission; an action potential in one neuron always leads to an action potential in the connected neuron
electrical synapses are relatively rare in the human nervous system,
e.g., a tooth’s pulp and the eye’s retina.
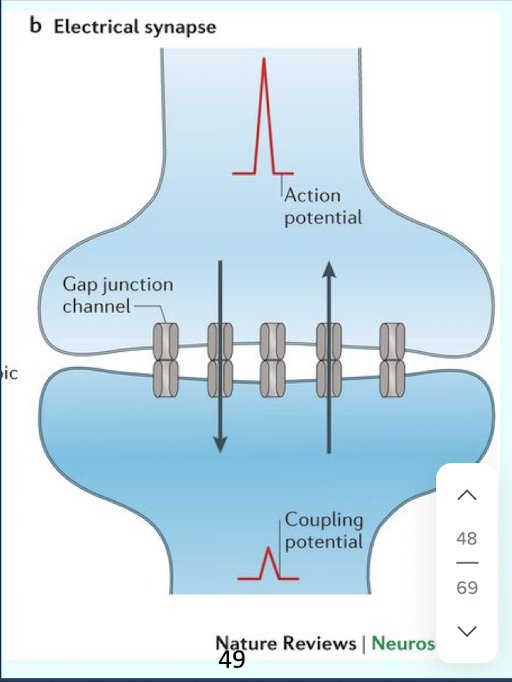
Which type of synapse represents the vast majority in the human nervous system?
Chemical synapse
How is information transmitted across a chemical synapse?
A chemical messenger (neurotransmitter) transmits information one way across a space separating the two neurons
What is the synaptic cleft?
The space between the presynaptic and postsynaptic neurons
In what direction do chemical synapses operate?
One direction only; the presynaptic neuron elicits changes in the membrane potential of the postsynaptic neuron; but the postsynaptic neuron does not directly influence the potential of the presynaptic neuron
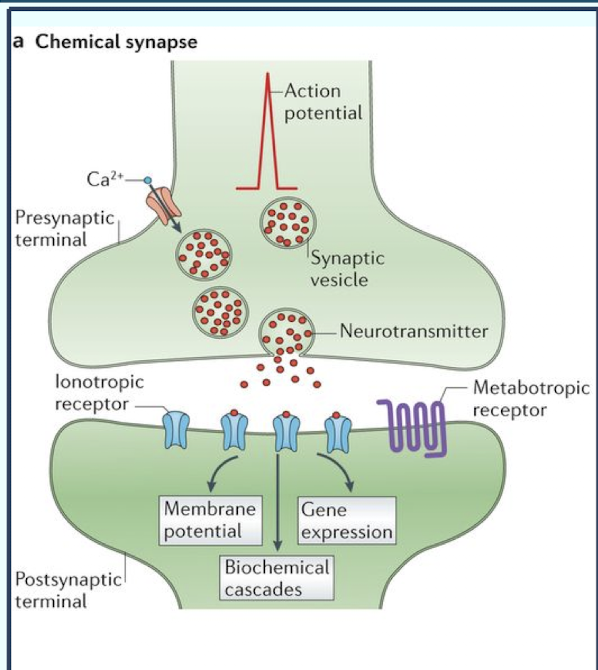
What is Synaptic Delay?
The time required for neurotransmitter release (via exocytosis) and receptor binding
What triggers the release of neurotransmitter into the synaptic cleft?
The action potential reaching the axon terminal triggers voltage-gated Ca2+ channels to open; Ca2+ enters the synaptic knob; triggering exocytosis of neurotransmitters from synaptic vesicles
What are the two types of post-synaptic (graded) potentials at chemical synapses?
Excitatory post-synaptic potentials (EPSP); Inhibitory post-synaptic potentials (IPSP)
What ion movement characterizes an EPSP?
Na+ influx (inward flow of positive ions) resulting in depolarization toward threshold
What ion movement characterizes an IPSP?
K+ efflux (outward flow of positive ions) or Cl- influx (inward flow of negative ions) or efflux; resulting in hyperpolarization or stabilization away from threshold
Can a single neurotransmitter produce different effects in different synapses?
Yes; neurotransmitters (e.g.; norepinephrine) can produce EPSPs at one synapse and IPSPs at another; depending on the subsynaptic receptor-channels
Which common neurotransmitters are primarily excitatory?
Glutamate; Aspartate
Which common neurotransmitters are primarily inhibitory?
Glycine; Gamma-aminobutyric Acid (GABA)
How do EPSPs and IPSPs compare to Action Potentials regarding magnitude and summation?
EPSPs and IPSPs are graded potentials; unlike action potentials (which follow the all-or-none law); graded potentials can be of varying magnitude; have no refractory period; and can be summed (added/subtracted to/from one another)
What two forms of summation influence the postsynaptic potential?
Temporal summation and Spatial summation
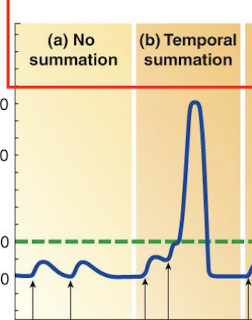
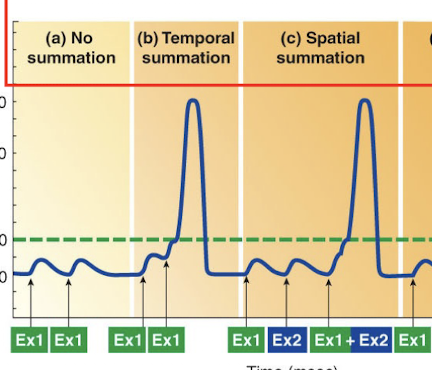
Describe Temporal Summation.
If an excitatory presynaptic input (Ex1) is stimulated a second time before the first EPSP in the postsynaptic cell has died off; the second EPSP will add onto or sum with the first EPSP; potentially bringing the postsynaptic cell to threshol
Describe Spatial Summation.
The postsynaptic cell may be brought to threshold by simultaneous activation of two (Ex1 and Ex2) or more excitatory presynaptic inputs; adding their effects togethe
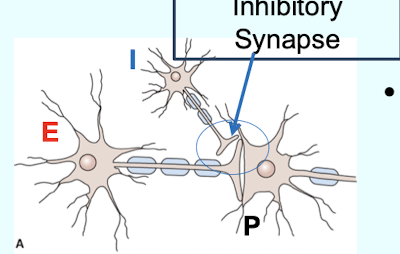
What is Presynaptic inhibition (indirect inhibition)?
Inhibition mediated by inhibitory neurons (I) whose terminals are on excitatory nerve endings; forming axo-axonic synapses and reducing neurotransmitter release from the excitatory neuron (E) onto the post-synaptic neuron (P)
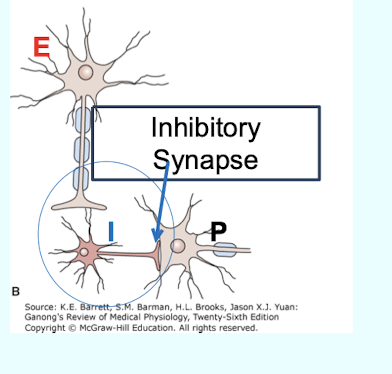
What is Postsynaptic inhibition (direct inhibition)?
Inhibition that occurs when an inhibitory transmitter; such as GABA; is released from the nerve terminals of an inhibitory interneuron (dark) that synapses with the postsynaptic neuron (P
What is the neuromuscular junction (NMJ), and how is it formed? What is the motor end plate?
The NMJ is a specialized junction where a motor neuron communicates with a muscle fiber. As a motor axon approaches a muscle, it branches, loses its myelin, and forms enlarged knoblike axon terminals (terminal buttons). Each terminal button sits above a specialized region of the muscle membrane called the motor end plate. Together, the axon terminal and motor end plate form the NMJ
What neurotransmitter is stored in the thousands of vesicles within each terminal button at the neuromuscular junction (NMJ)?
Acetylcholine (ACh)
What triggers the release of ACh by exocytosis into the cleft at the NMJ?
An action potential in motor neurons triggers the opening of voltage-gated calcium (Ca2+) channels in all terminal buttons; Ca2+ diffuses into the terminal button from its higher extracellular concentration
What happens when ACh binds to receptors on the motor end plate (Step 5)?
The binding brings about the opening of nonspecific cation channels; leading to a relatively large movement of Na+ into the muscle cell compared to a smaller movement of K+ outward

What enzyme destroys ACh after it acts on the motor end plate?
Acetylcholinesterase; which removes ACh from the motor end-plate membrane; terminating the muscle cell's response
What type of potential is the End Plate Potential (EPP)?
A graded potential; similar to an EPSP; but much larger
Why is the EPP much larger than a typical EPSP?
Reasons include:
1) NMJ consists of multiple terminal buttons simultaneously releasing ACh (more release sites);
2) More neurotransmitter is released per terminal button;
3) The motor end plate has a larger surface area and higher density of receptor-channels (more receptors);
4) Many more receptor-channels are opened; permitting a greater net influx of positive ions and a larger depolarization
Can an action potential be initiated directly at the motor endplate region?
No; the motor endplate region does not have a threshold potential; so an action potential is not initiated at this site
How does the EPP generate an action potential in the muscle fiber?
The EPP causes current to flow between the depolarized end plate and the adjacent resting cell membrane; this flow causes the opening of voltage-gated Na+ channels; reducing the potential to threshold in adjacent areas; leading to a subsequent AP that propagates throughout the muscle fiber by contiguous conduction
Compare the general transmission ratio of an AP at the NMJ versus a typical postsynaptic neuron synapse.
One-to-one transmission of action potentials occurs at a neuromuscular junction (one presynaptic AP usually guarantees a postsynaptic AP); whereas a postsynaptic neuron typically requires summation of EPSPs to bring the membrane to threshold
Is a neuromuscular junction always excitatory?
Yes; a neuromuscular junction is always excitatory (an EPP); whereas a synapse may be either excitatory (an EPSP) or inhibitory (an IPSP)
Where does inhibition of skeletal muscles occur; since it is not accomplished at the NMJ?
It can occur only in the CNS through IPSPs at the dendrites and cell body of the motor neuron