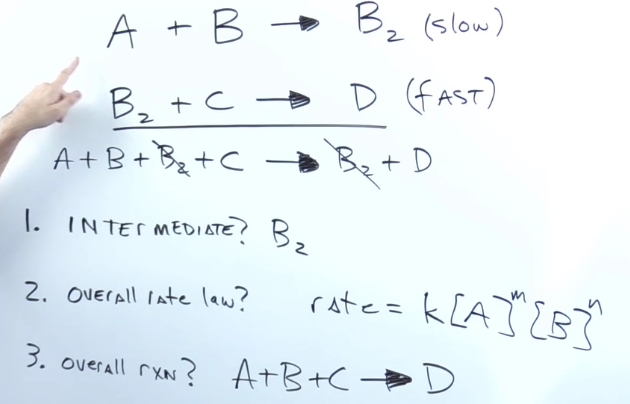Chemistry - Chemical Kinetics
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Rate expression
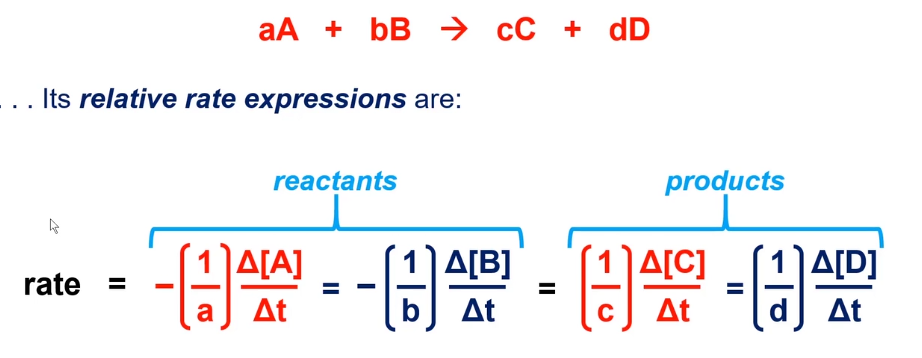
Rate law
m and n must be determined experimentally
Find where rate changed in one and stayed the same in the other
divide rate and concentration
set concentration to the power of m equal to rate
concentration^ m = rate ; solve for m
do the same for other variable
Zero order linear graph
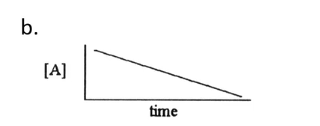
First order linear graph
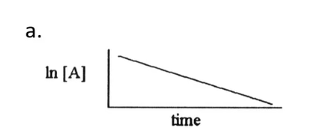
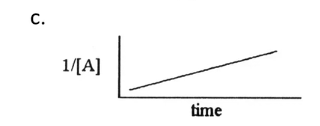
Second order linear graph
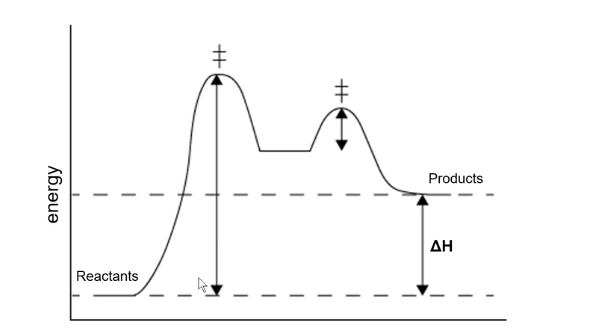
Delta H Graph; Endo or Exothermic?; Where is transition states and intermediate?; Where is Ea1 and Ea2?
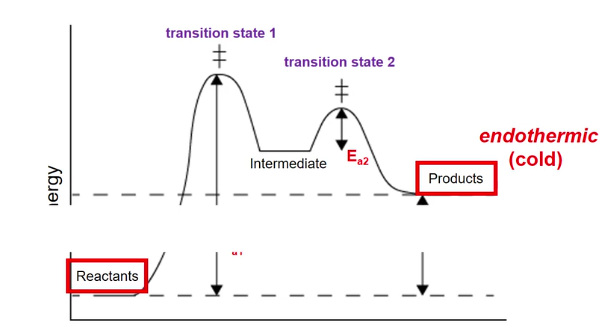
What is the rate determining step; how do you see it in the Delta H graph?
slowest step
The step with the largest Ea
Are catalysts consumed in a reaction?
no they are regenerated
What are protein catalysts called?
Enzymes
How do catalysts speed up a reaction?
By lowering a reactions activation energy (Ea)
(not by changing the energy levels of the reactants or products)
How do catalysts lower Ea?
by providing an alternative pathway (different mechanism) between reactants and products
Do catalysts shift equilibrium reactions?
no
Collision Theory
Molecules have to collide to react
The greater the number of collisions per second, the greater the chance that molecules will hit each other
Molecules must hit each other with at least Ea
Increasing temperature can increase a molecules speed
Must molecules hit each other is the correct 3 dimensional orientation?
yes
As temperature goes us, k (rate constant) and reaction rate go?
up and up

If K (rate constant) and reaction rate go up, Ea goes?
down
What is the rate determining step?
the slow step
When given elementary steps how do you find the intermediate?
substance produced in the middle of the reaction that get used up before the product
They get cancelled out along the way
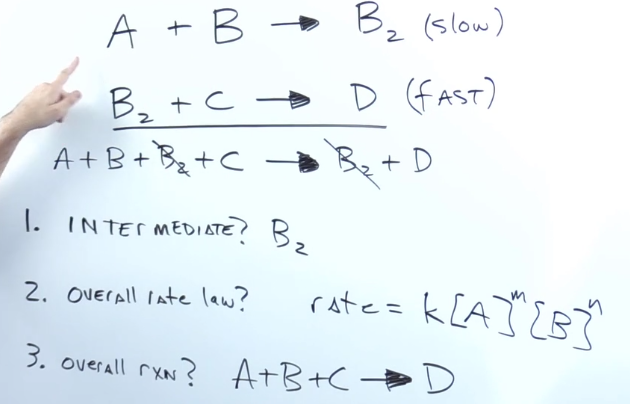
When given elementary steps, what is the overall rate?
Rate = k * the concentration of each reactant in the slow step multiplied by each other
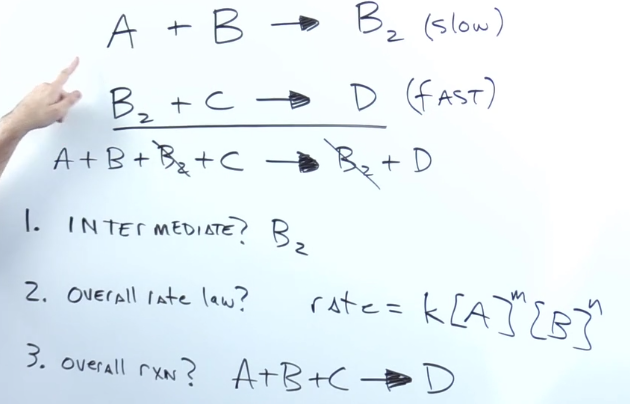
When given elementary steps, what is the overall reaction?
Add up elementary steps and cancel out intermediates