Units 1.1 & 1.3 Formulae & Equations + Chemical Calculations
1/87
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
88 Terms
What do the following state symbols mean? (s, l, g & aq)
(S) - Solid
(L) - Liquid
(G) - Gas
(Aq) - Aqueous ~ dissolved in water (solution) e.g NaOH(aq)
What is a compound ending in -ide and in -ate?
-ide - metal + non metal
-ate - metal + non metal + oxygen
What is the difference between an element and a compound?
Element - contains only one type of atom
Compound - contains two or more different atoms chemically combined
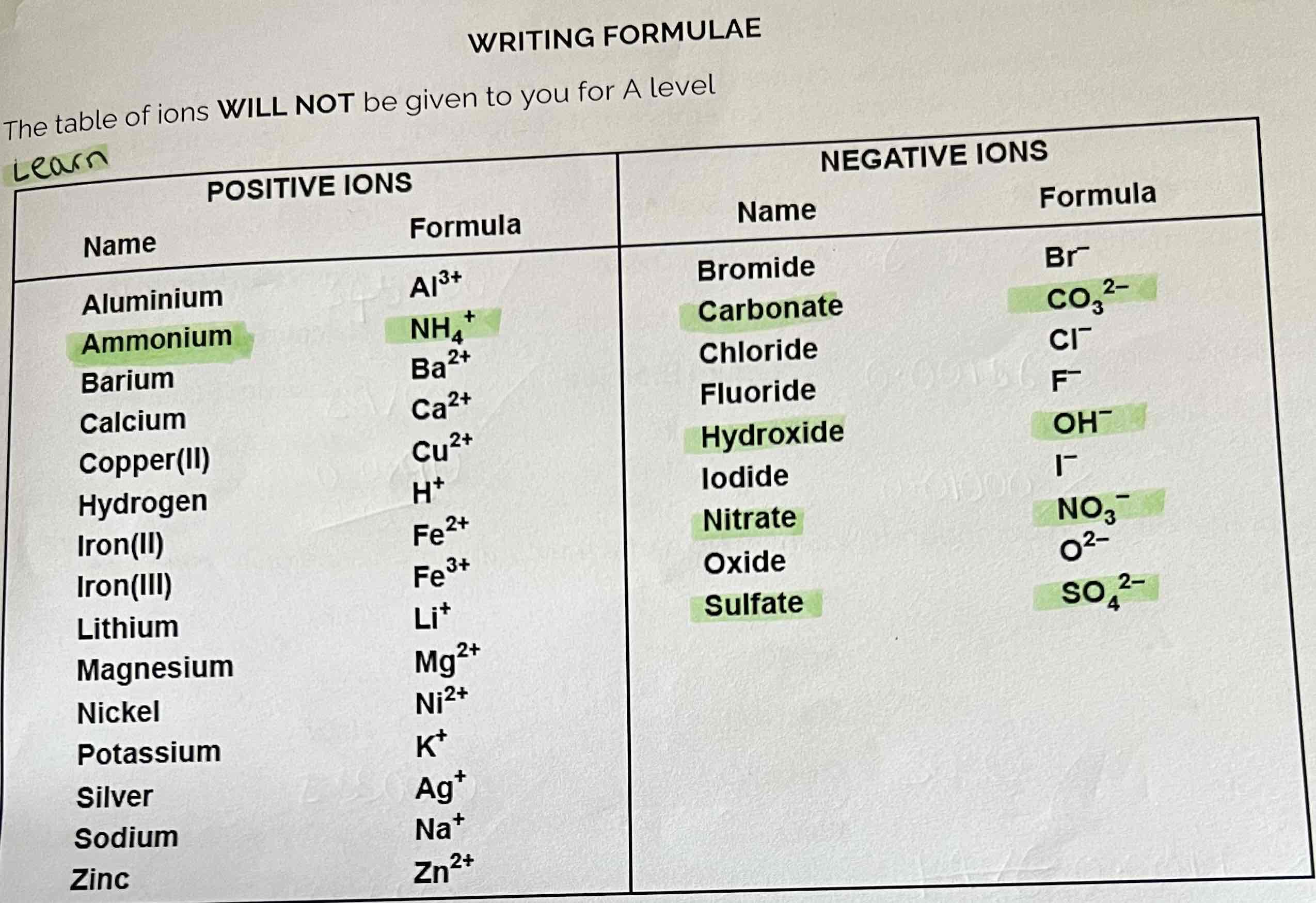
The Table of Ions
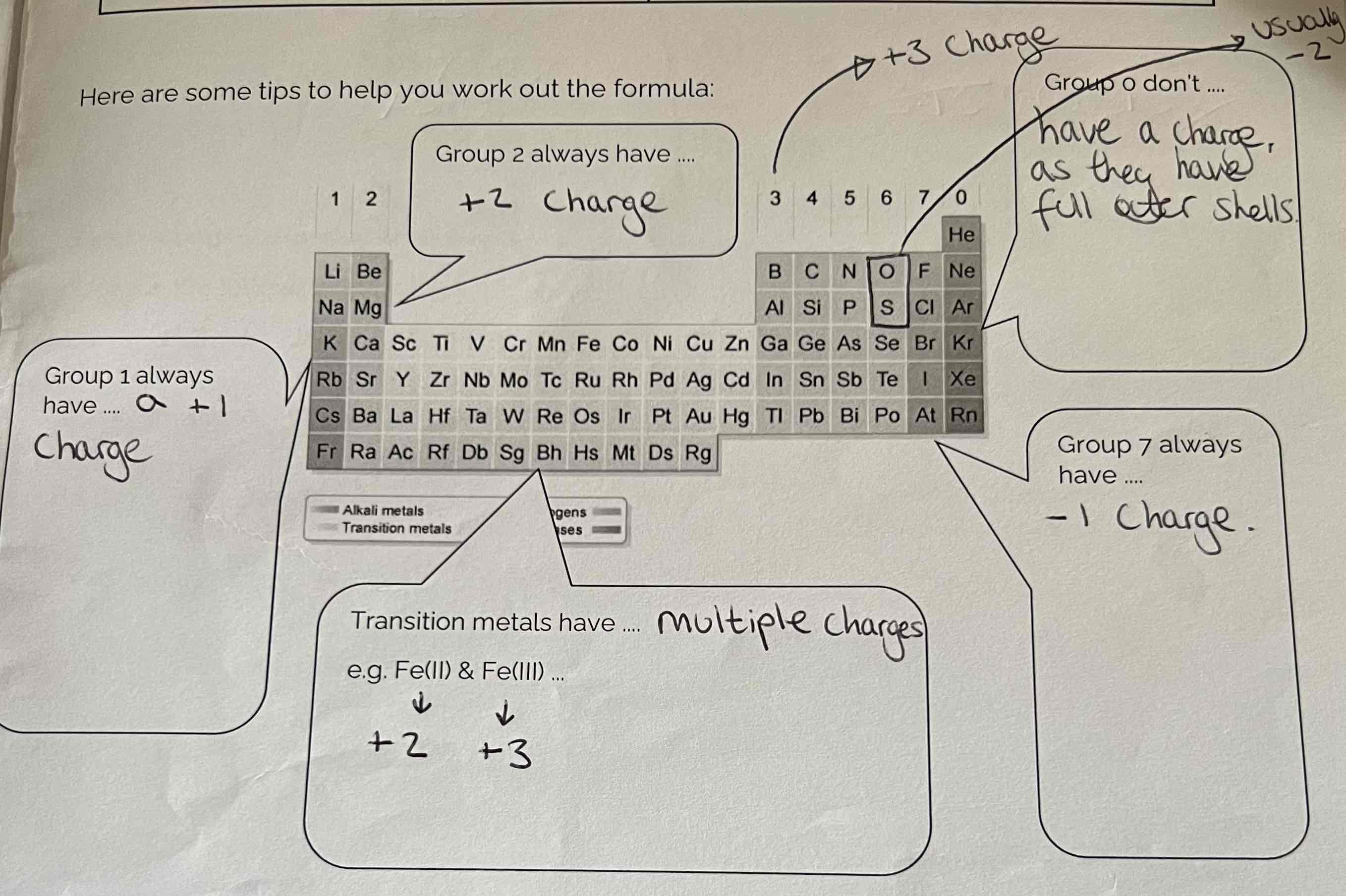
Tips to help work out the formula/ ions using the periodic table
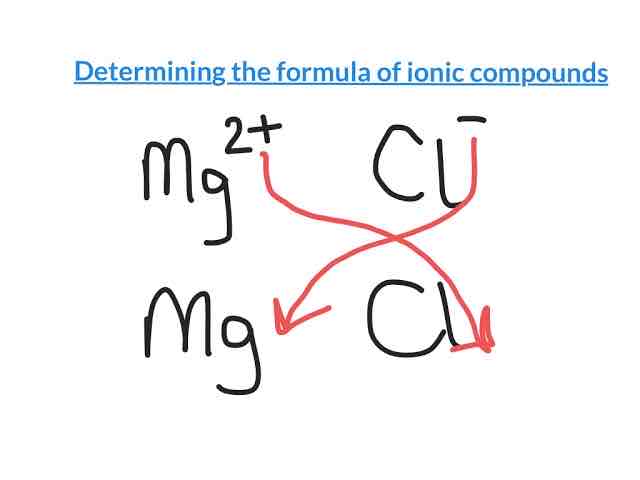
How do we determine the formula of Ionic Compounds?
We use the drop & swap method
In this example, the formula would be MgCl2
Remember: Metal always comes first
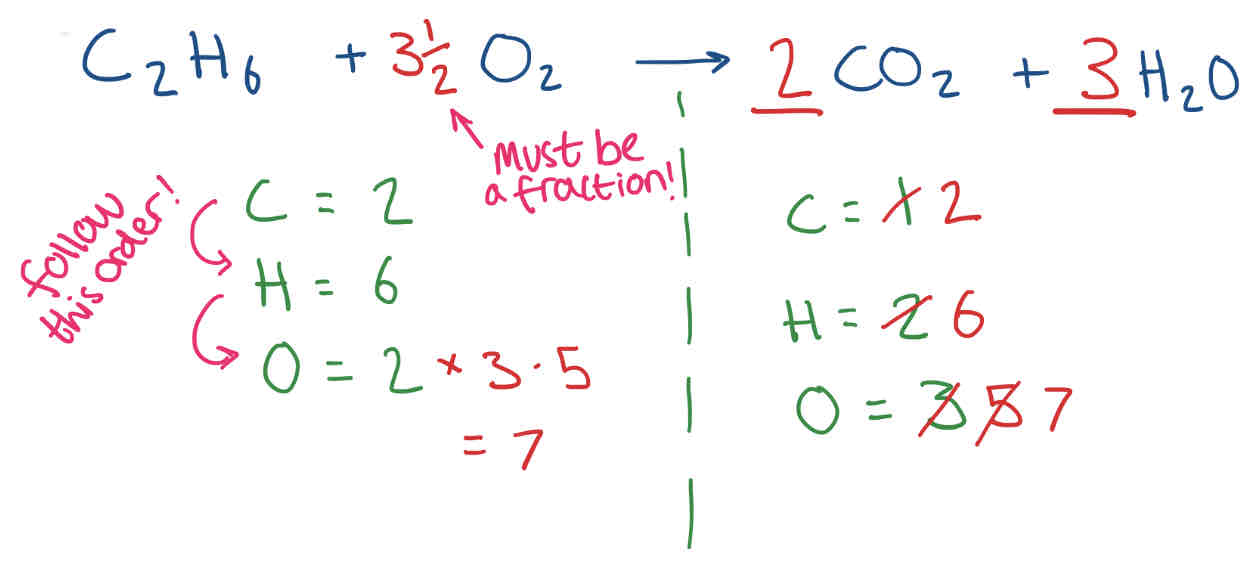
Balancing Equations
When balancing equations, balance the least common elements first. Then, work your way up to the most common elements in the equation.

ii) to ensure that all the carbonate ions were precipitated
d) some of the carbonates decompose / evolve CO2 on heating (1)
this will result in a lower mass being recorded (1)
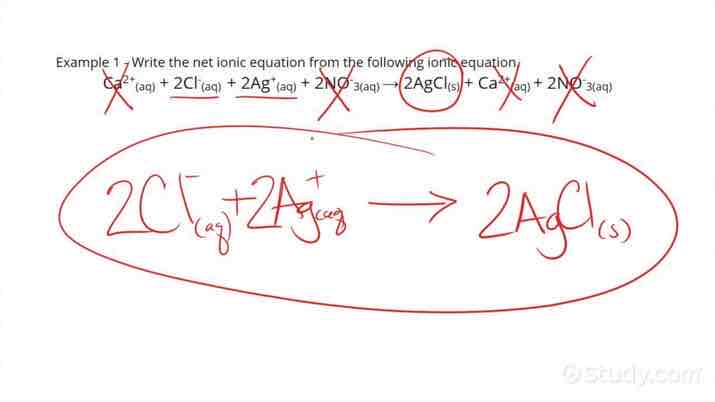
Ionic Equations
Ionic equations show the ions that are taking part in the reaction that produce the precipitate (they don’t show spectator ions).
What is Oxidation/ Reduction?
Oxidation is loss of electrons.
Reduction is gain of electrons.
Remember: OIL RIG
What do Oxidising/ Reducing agents cause?
Oxidising agents cause oxidisation in another element.
Reducting agents cause reduction in another element.
What is a redox process?
Reaction that has both reduction and oxidation occurring
and
Show change in oxidation state
What is an Oxidation Number/ State?
An oxidation number is a number assigned to an atom to describe its relative state of oxidation or reduction i.e the number of electrons lost or gained by that atom after it is transformed form its stable elementary state (always = 0 oxidation number)
Why is the movement of electrons important and what causes it?
Electron movement is important as all chemical reactions are based on the movement of electrons.
Without electron movement there would be no chemical reactions and no new substances made other than the elements in the periodic table.
Electronegativity causes electron movement.
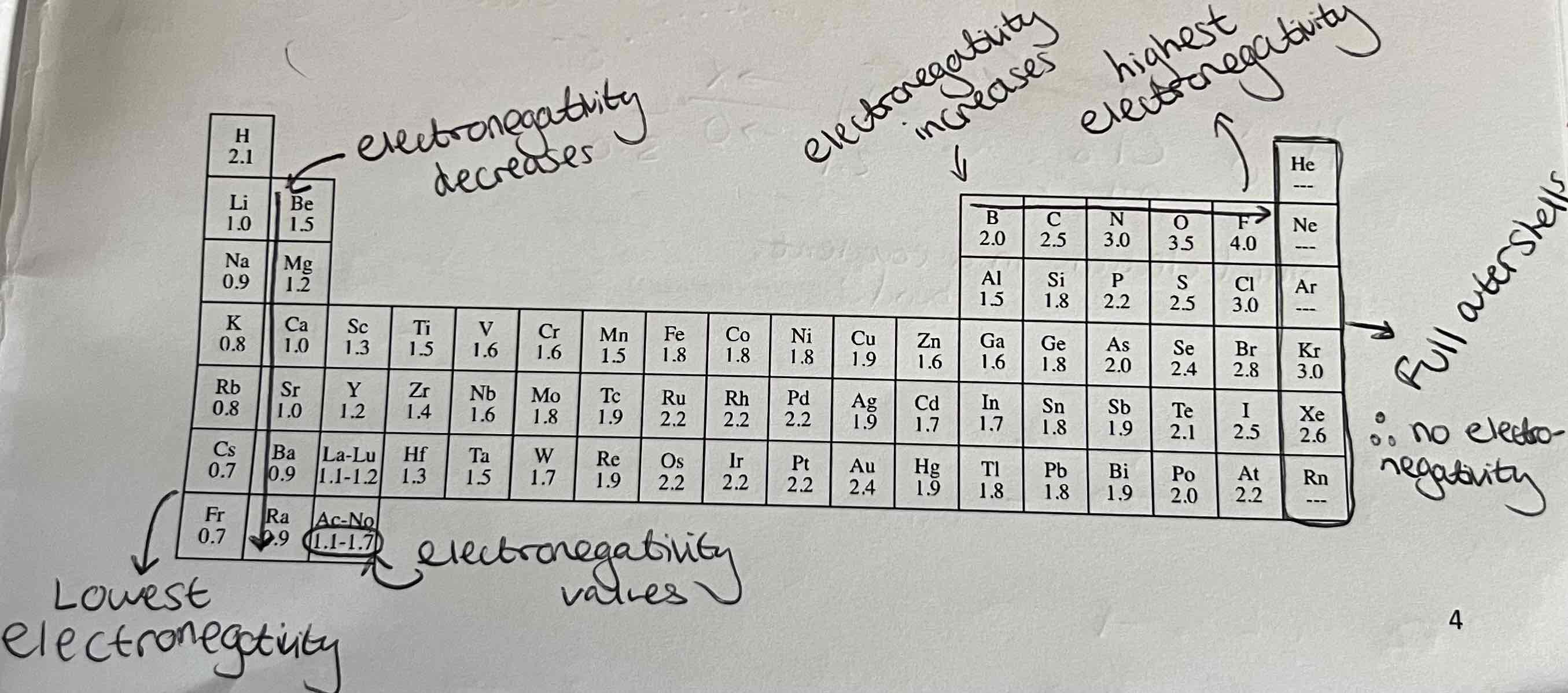
What is the definition of Electronegativity?
The ability of an atom to attract electrons towards itself
Electronegativity Trends, Patterns & Values in the Periodic Table
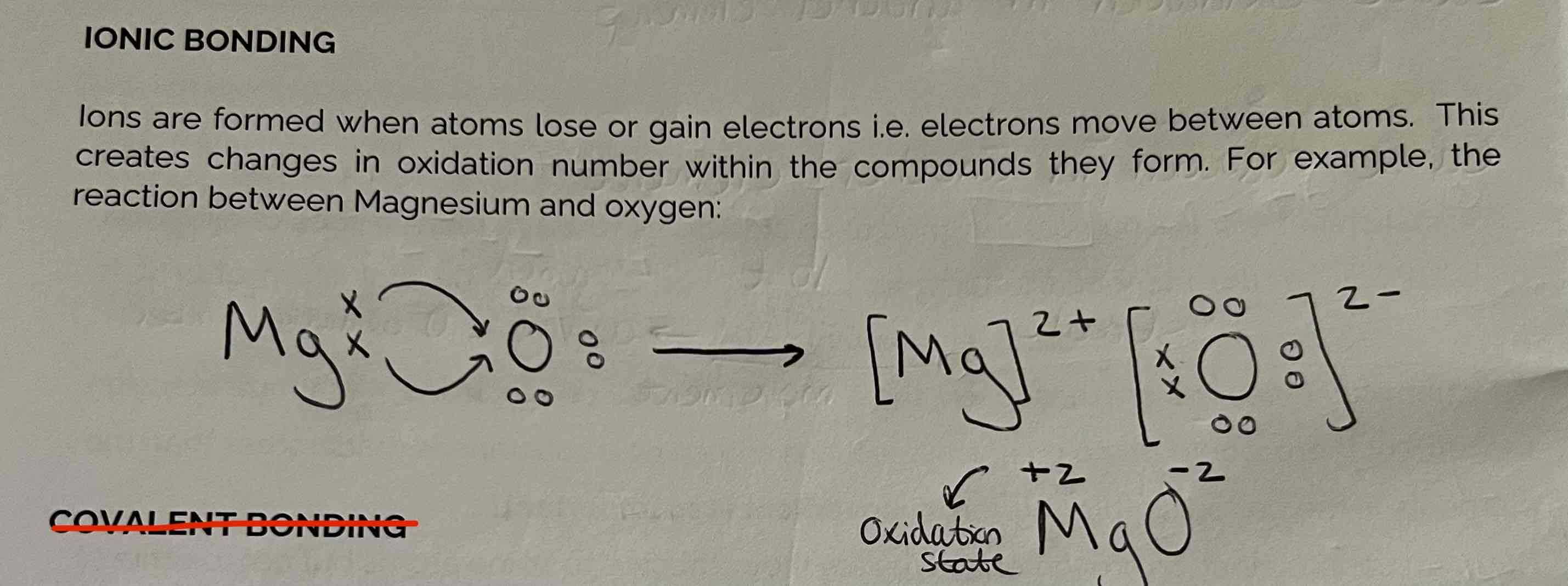
Oxidation State in Ionic Bonding Example
The charge is equal to the oxidation state.
Electronegativity in Covalent Bonding + Example
Differences in Electronegativity cause dipoles which are differences in charge of atoms either side of a covalent bond.
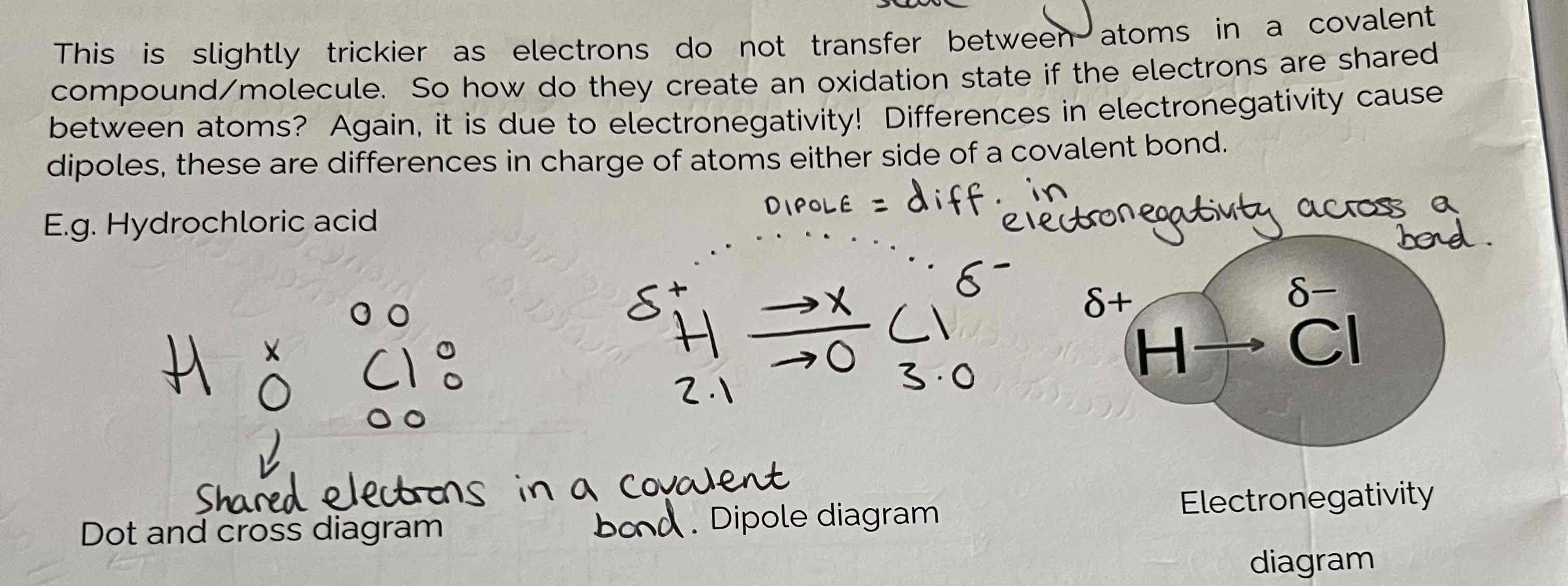
Dipoles Examples
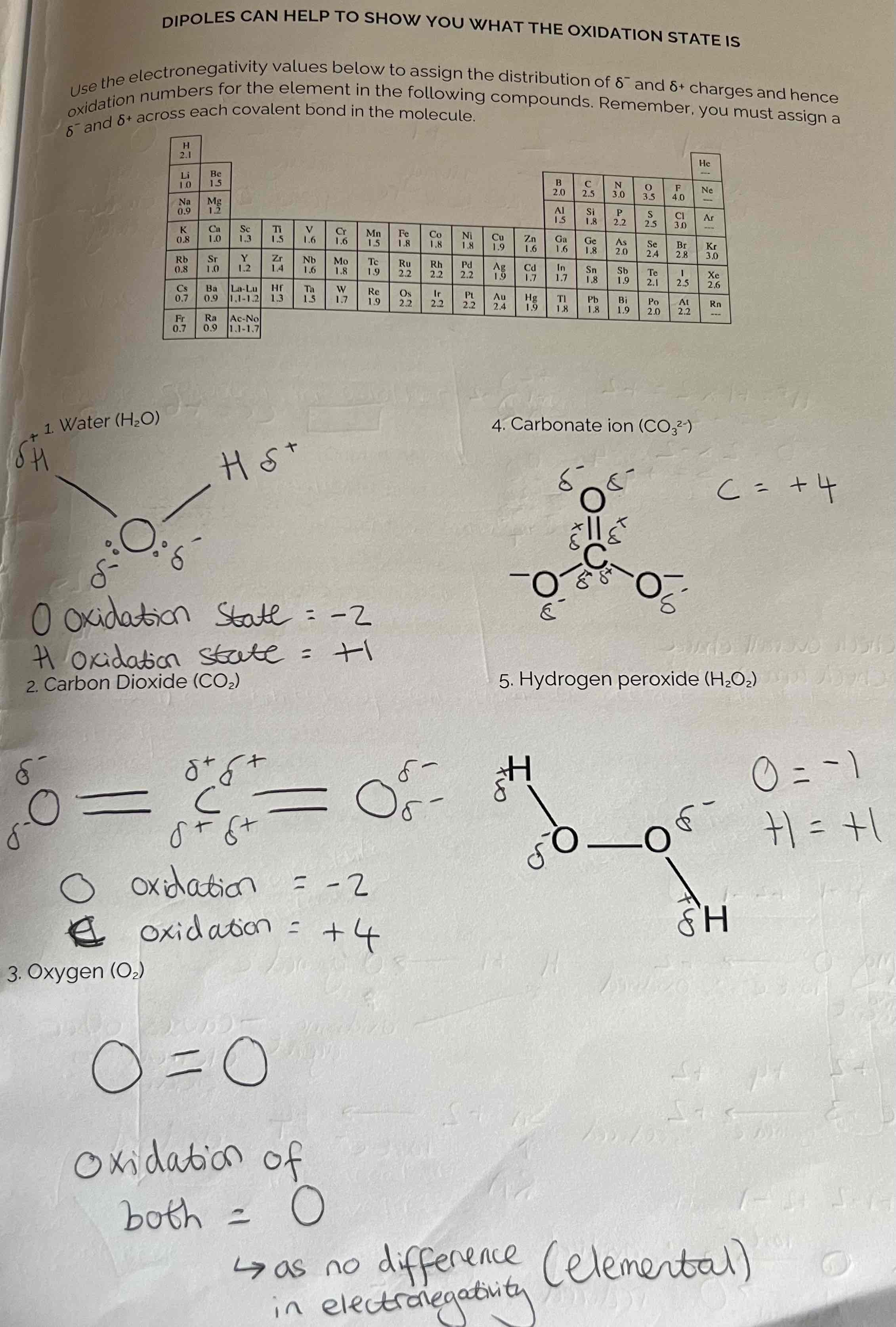
Oxidation States to learn
Oxidation state of certain elements that apply 99% of the time:
Hydrogen - +1 as it always loses 1e-
Oxygen - -2 as it gains 2e- (except in H2O2 it is -1)
G1 Elements - +1 as they always lose 1e-
G2 Elements - +2 as they always lose 2e-
G7 Elements - -1 as they always gain 1e-
Elements - 0 oxidation state as there is no difference in Electronegativity
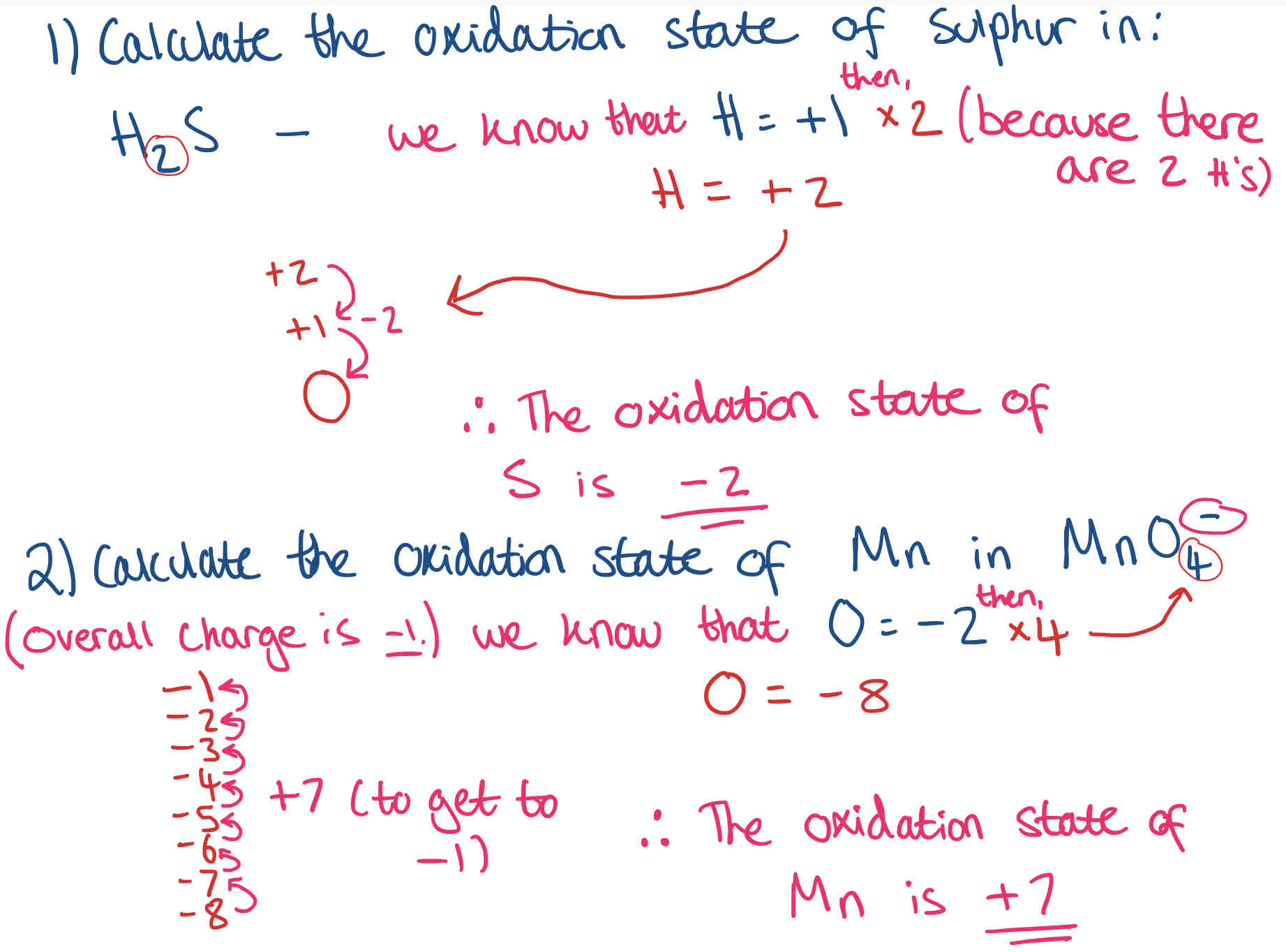
How do we calculate oxidation states? + Examples
Check overall charge
Check known elements
How much needs to be added/ taken away to get to 0/ overall charge - this is the oxidation state (remember to divide element your trying to find if there’s more than one of them - to get one)
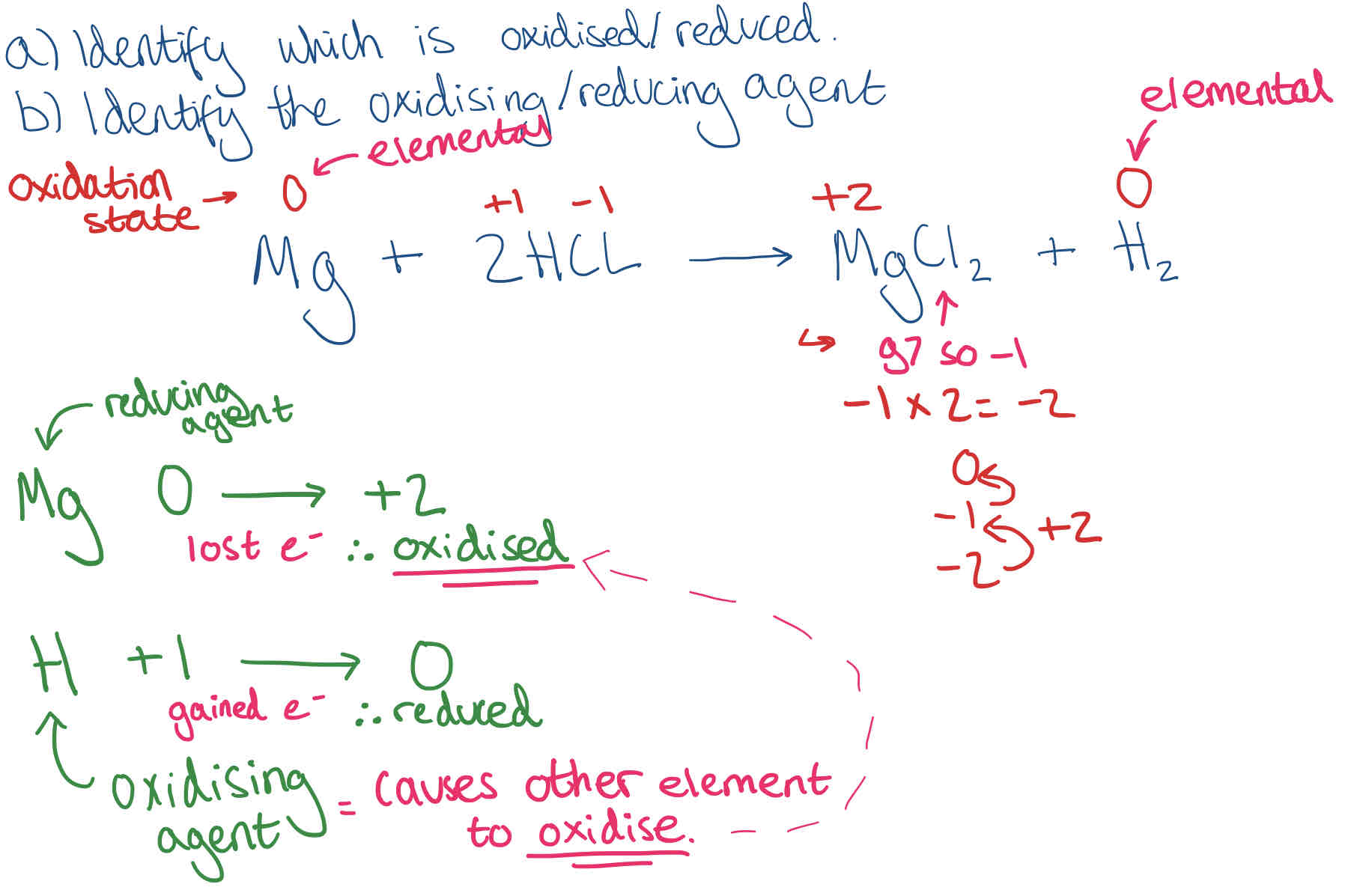
Oxidising/ Reducing agents in equations
Find oxidation states for each element/ compound in the equation (ignore numbers)
Remember: Elements on their own have 0 oxidation state
Show the change in oxidation state, then state whether the element has oxidised or reduced.
State which element is the oxidising/ reducing agent (opposite of whether it has been oxidised/ reduced)
What is the definition of Relative Atomic Mass (Ar)
The ratio of the average mass of an element to 1/12 the mass of a C-12 isotope.
What is the Relative Isotopic Mass?
The mass of the most common isotope.
How do you work out the Relative Molecular/ Molar Mass (Mr)?
To work out the Mr (mass of the whole compound) all you have to do is add up all the relative atomic masses (top number) to give you the formula mass of the whole compound.
Remember to include the decimal points.
Percentage Composition Calculations + Example
% composition = mass of element/ total Mr x 100
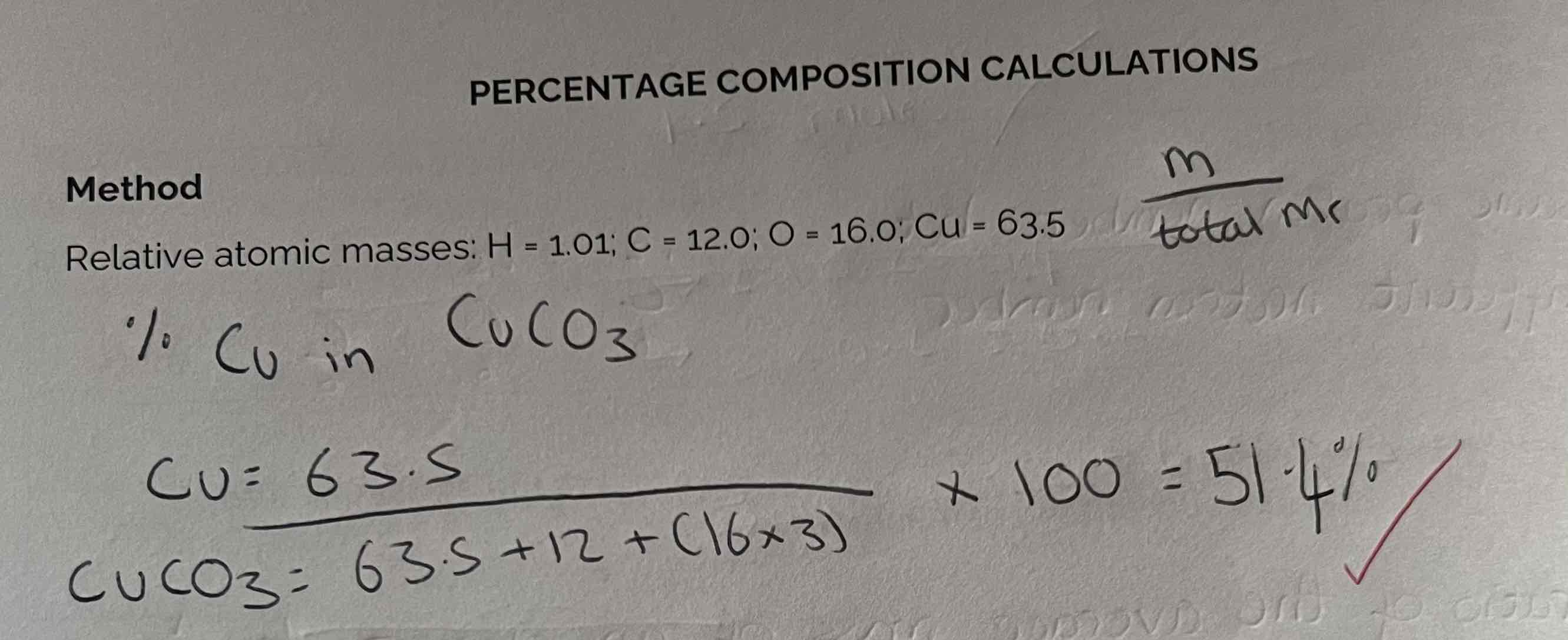
What is the definition of The Mole?
The amount of substance that contains the same number of particles as there are atoms in 12g of C-12.
What is One Mole?
One mole of any substance contains 6.02 x1023 particles. This is a very large number and is known as Avogadro's constant (L). The relative atomic mass in grams of any element contains 1 mole of atoms.
Remember:
• One mole = 6 x1023 particles
• So half a mole = 3 x1023 particles
• 2 moles = 12 x1023 particles
How do you work out how many particles are present in a compound?
no. of moles x Avogadro’s Constant
e.g 6 moles of Na = 6 × 6.02 ×1023
If you have 1 mole of Copper Sulphate, CuSO4, how many moles of oxygen atoms do you have?
4 moles as there are 4 O’s
If you then had 0.5 moles of Copper Sulphate, CuSO4, how many moles of oxygen atoms do you have?
2 moles - 0.5 × 4
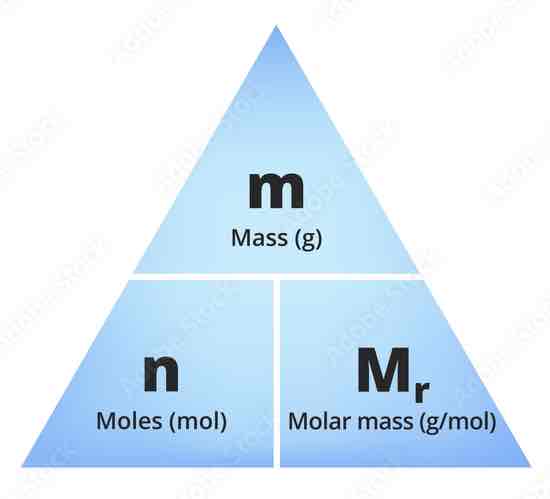
Calculating involving moles - What is the Formula?
No. of moles = Mass (g)/ Mr (g/mol)
Example of mole calculations
Molecules - x by Avogadro’s
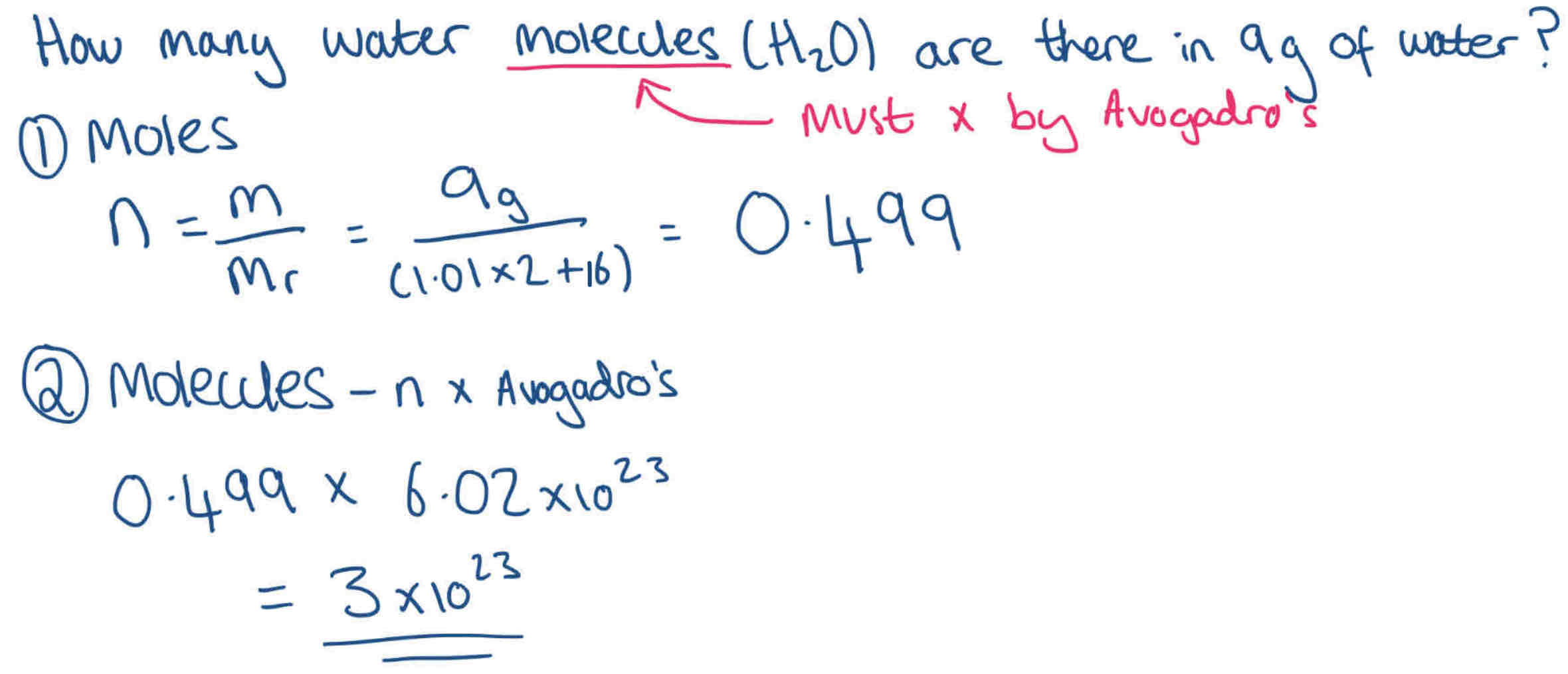
What is the Theoretical Yield & How do you work it out? + Example
The theoretical yield is the mass of product that could be made.
Steps to work out:
Find Mr of both compounds (ignore num. in front as we will check ratio after)
no. of moles
Check ratio & multiply or divide if necessary
mass
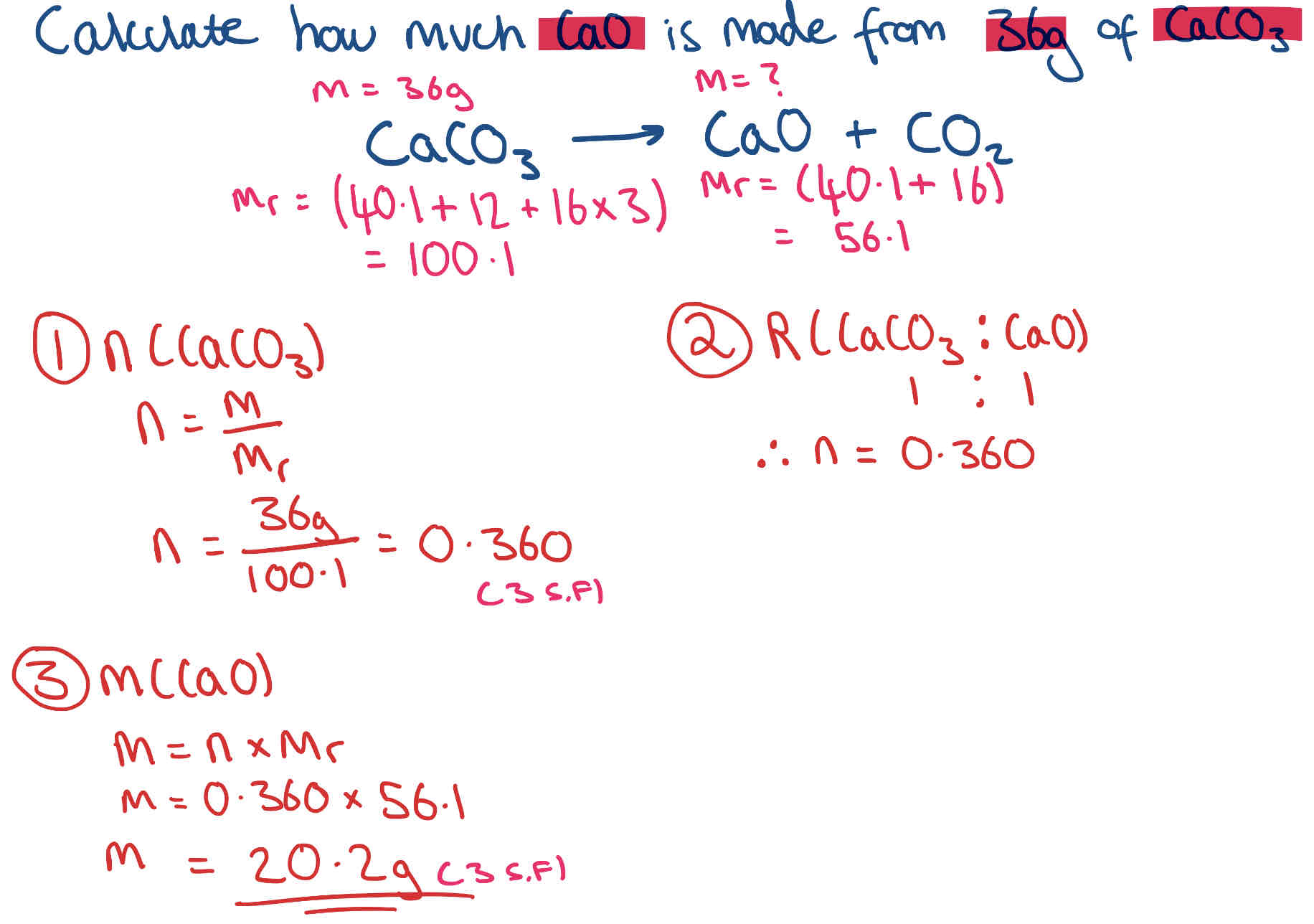
What is the stochiometry?
The relationship of number of moles of reactants to those products is called the stochiometry of the reaction.
An equation giving the correct stochiometry is called a balanced equation.
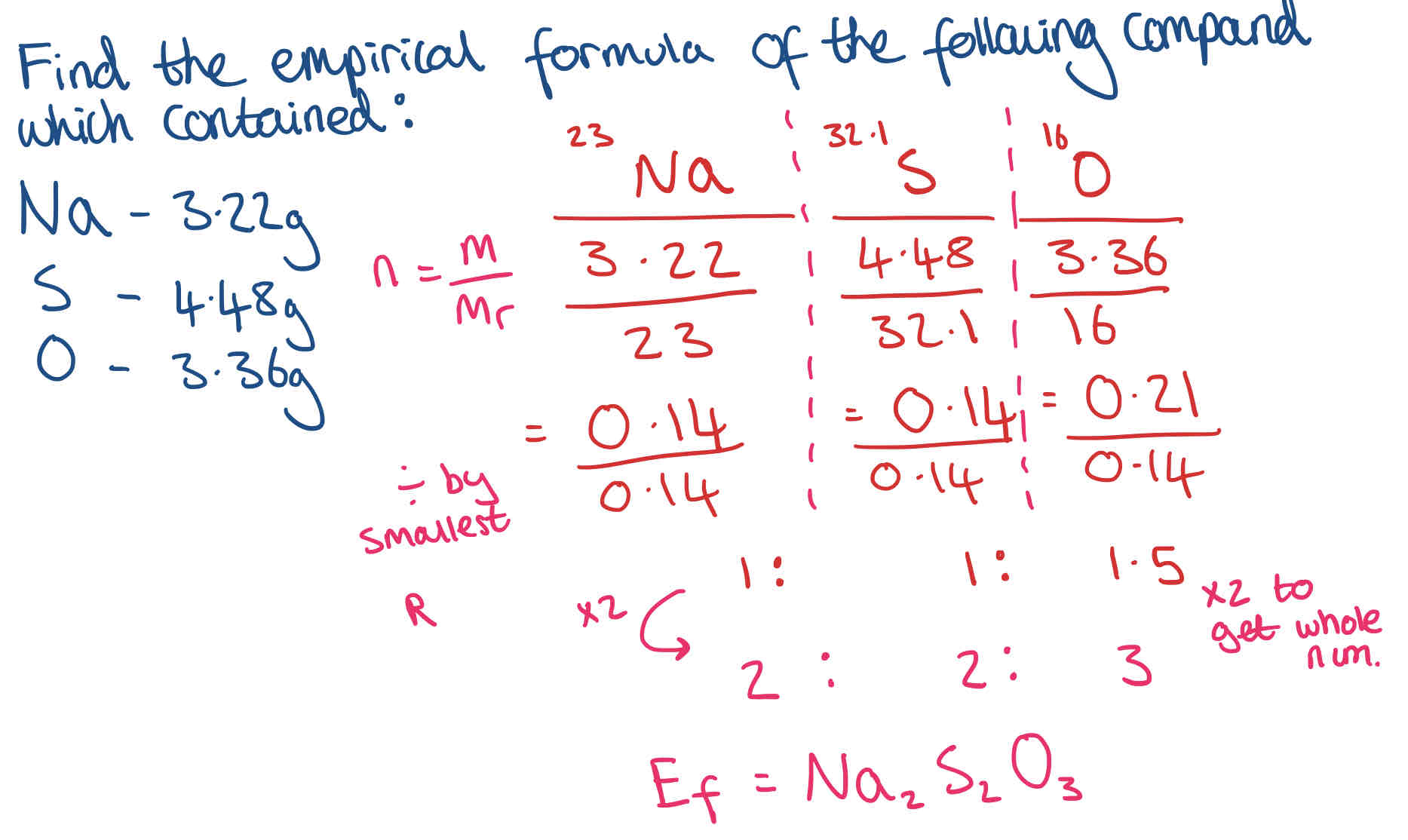
What is the Empirical (Simplest) Formula & How do you work it out? + Example
The empirical formula of a substance is the simplest whole number ratio of elements within a compound. e.g. benzene has a molecular formula of C6H6, but it’s empirical formula is CH
To work out:
n = m (or percentage)/ Mr
Divide by smallest
Ratio is Formula (If answer is a decimal, multiply all numbers to get a whole number)
Can round to whole number if it is very close to it (0.999 ~ 1)
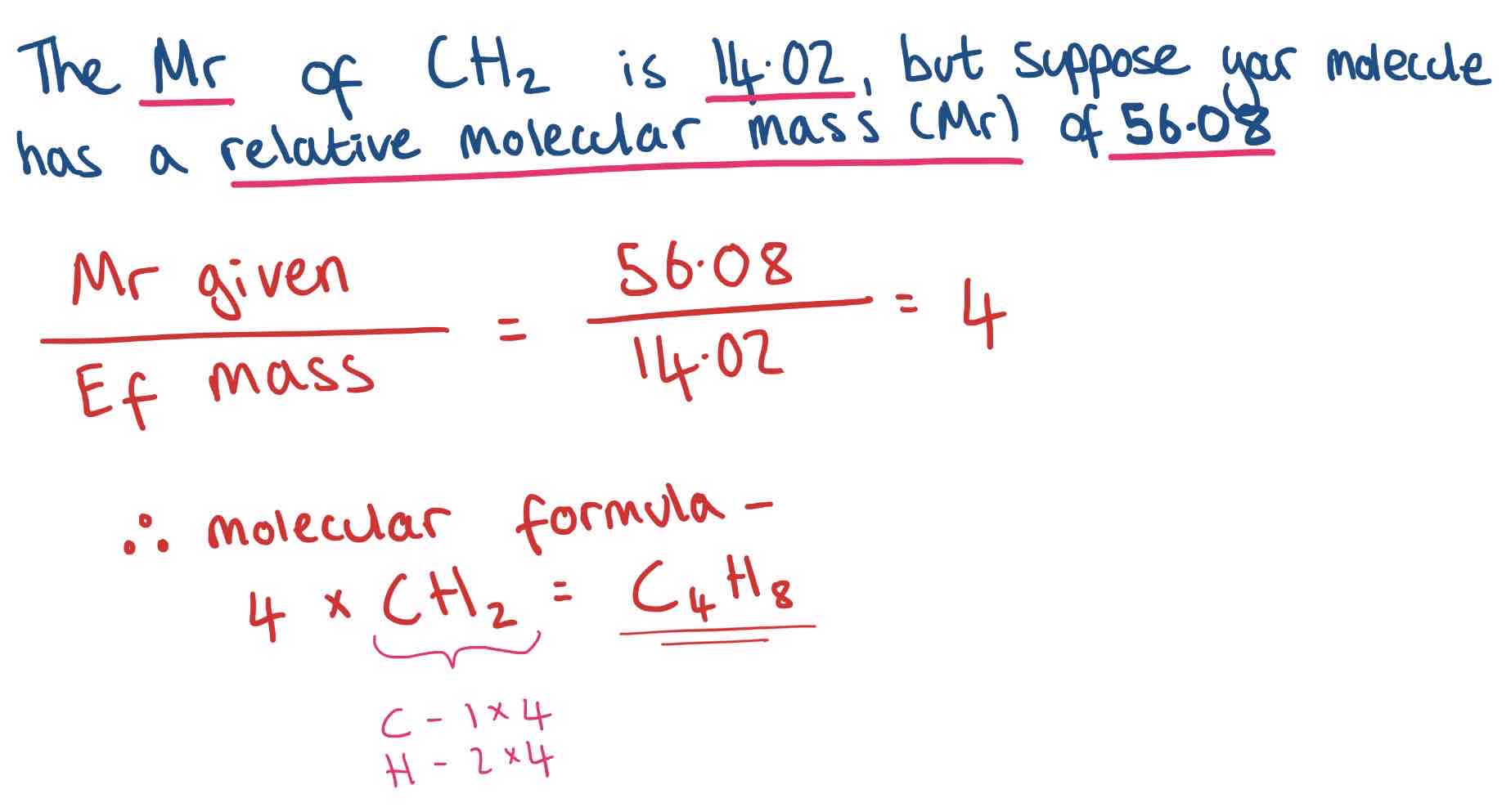
Converting Empirical Formula into Molecular Formula
The molecular formula can be found from the empirical formula if the relative molecular mass or molar mass of the compound is known.
Method to work out:
Divide Mr given/ Empirical Formula Mass
Multiply each of the small numbers in the compound by the number you just worked out
Determination of xH2O + Example
How much water of crystallisation is required or driven off during a reaction
Steps to work out x:
(Compound).xH2O = mass given
Work out mass of Compound (residue given) + H2O by taking away from total mass
n = m/ Mr
Divide by smallest
Ratio is formula - (Compound).XH2O
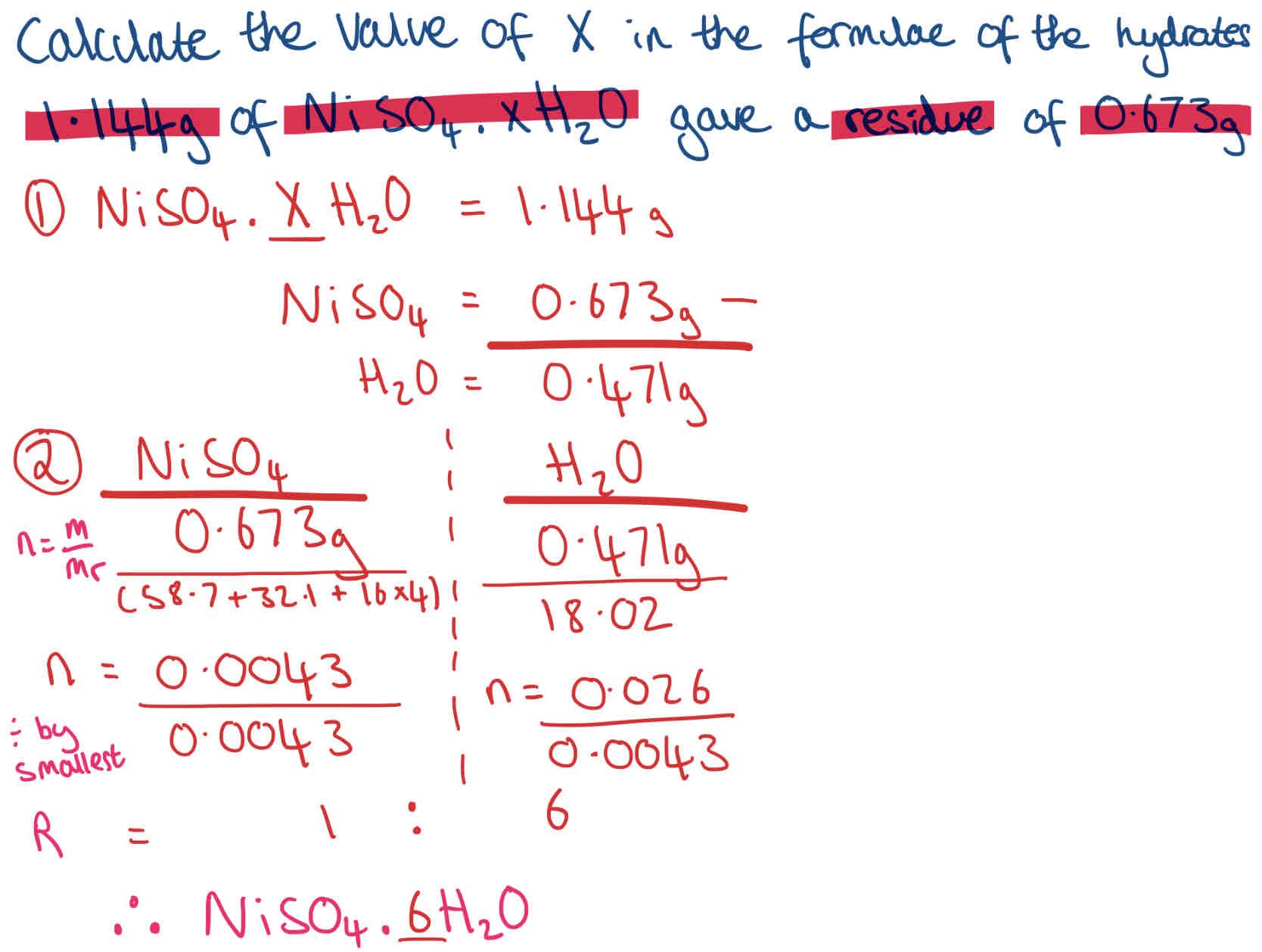
Suggest how a student doing a crystallisation experiment would ensure that all the water had been removed.
Heat to constant mass
How do you work out the percentage yield? + Example
% yield = actual/ theoretical x 100
In this example, you would work out:
no. of moles in ethanol = m/ Mr
Check ratio
mass of ethene (theoretical yield)
% yield = actual/ theoretical x 100

Why can’t percentage yields always be 100%?
The reaction is reversible - Products can become reactants again so reducing yield
Filtration - Product remains behind in the filter apparatus
Transferring liquids - Liquid/ residue remains in containers
Unexpected reactions - Alternative pathways means we get less of the desired produce
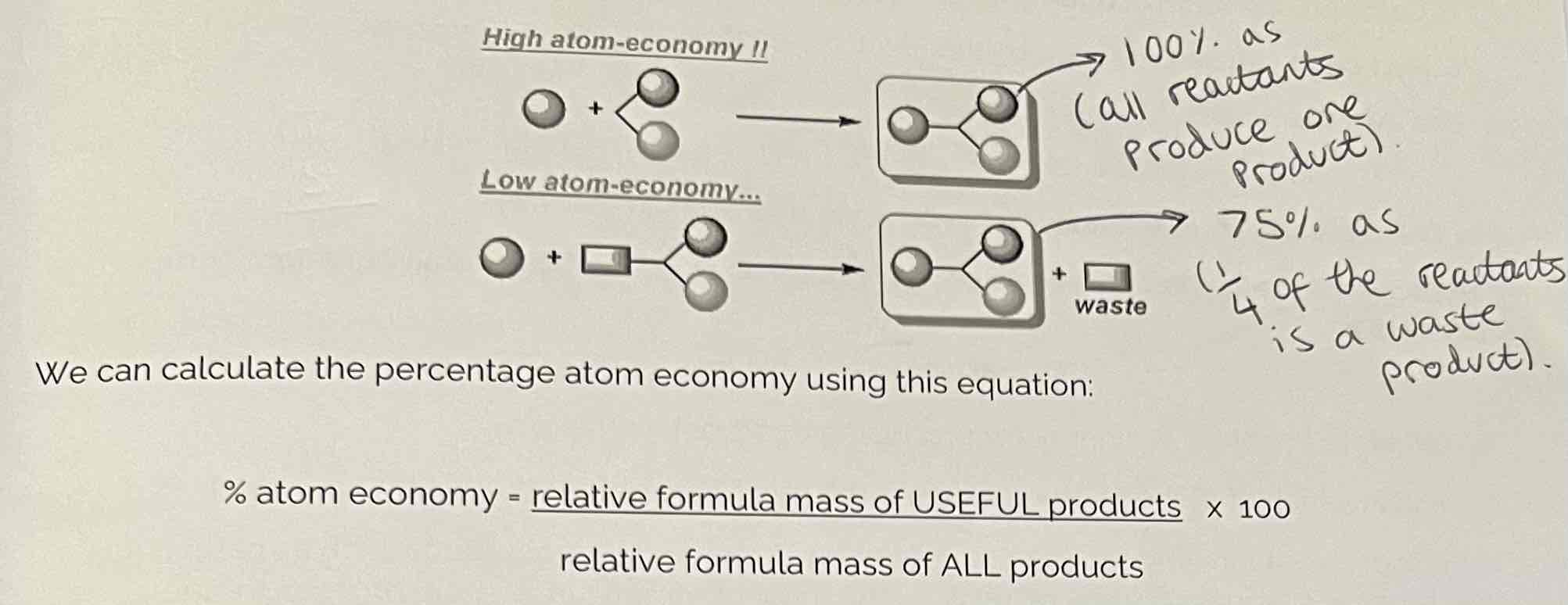
What is Atom Economy?
Atom economy (or atom utilisation) is a measure of the amount of starting materials that end up as useful products.
The careful management of resources is important for sustainable development and for economical reasons to use reactions with high atom economy.
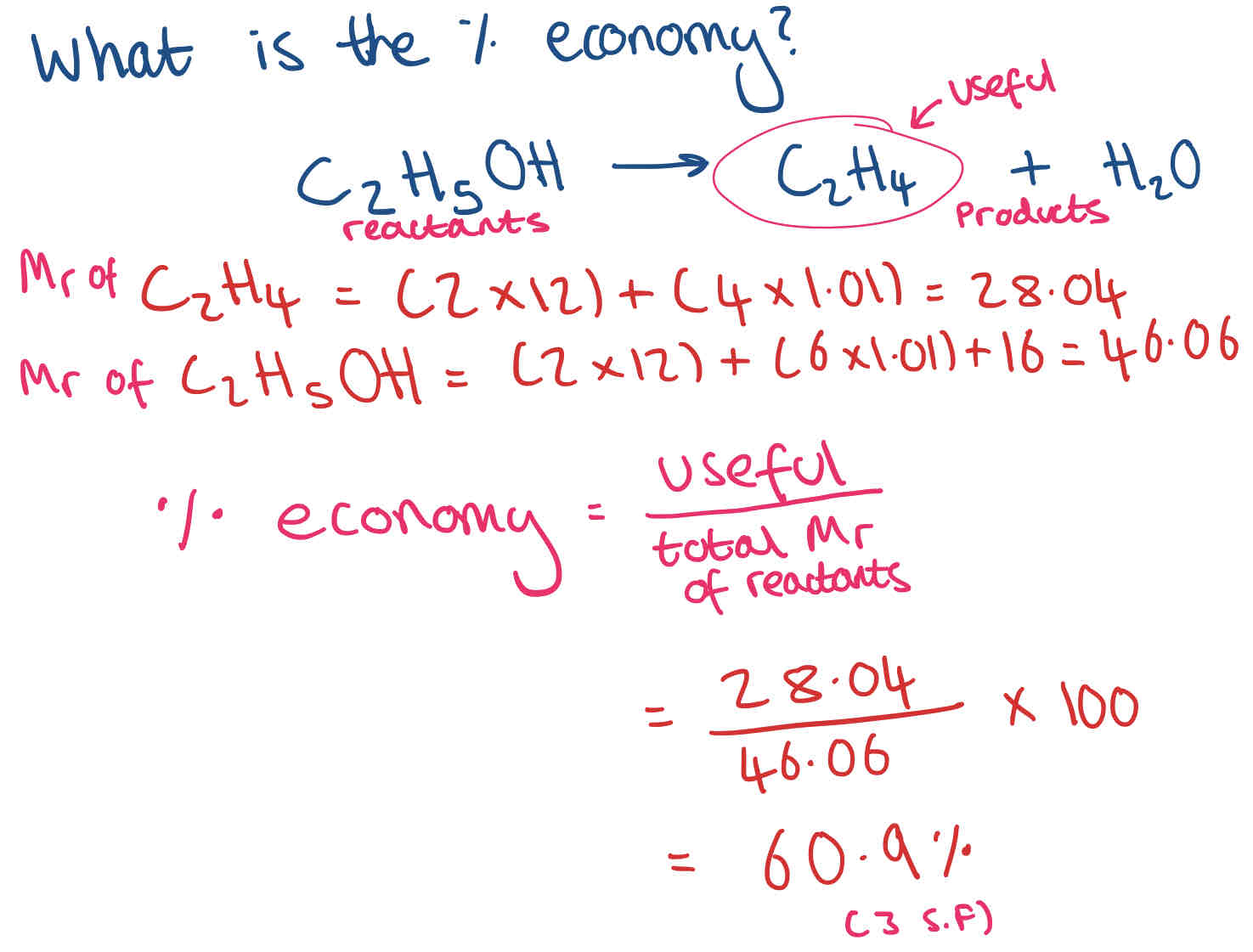
High & Low Atom Economy + How do we calculate the percentage atom economy?
% atom economy = useful/ total Mr of all reactants x 100
Include numbers in front when working out Mr for atom economy.
Example of % economy calculation
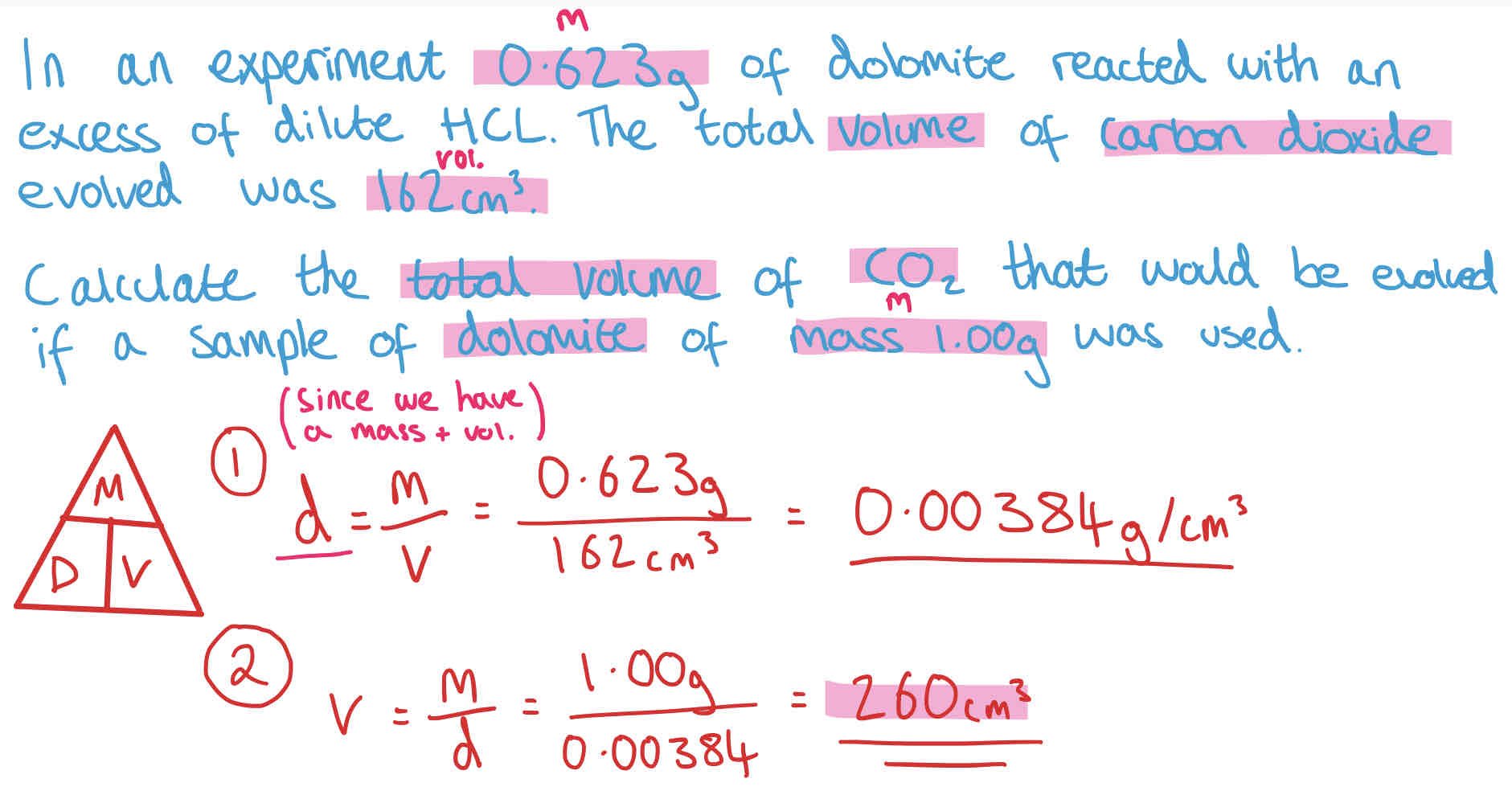
How to Calculate Density? + Example
Density measures the amount of mass per unit of volume in a substance.
Density can be found using the equation:
Density = mass/ volume
DMV
Converting Density to Concentration
Calculate the number of moles that you have in a solution using:
n = m/ Mr
Find the volume that this number of moles is dissolved in using:
D = m/ v
Find the concentration (molarity) using:
n = v x c
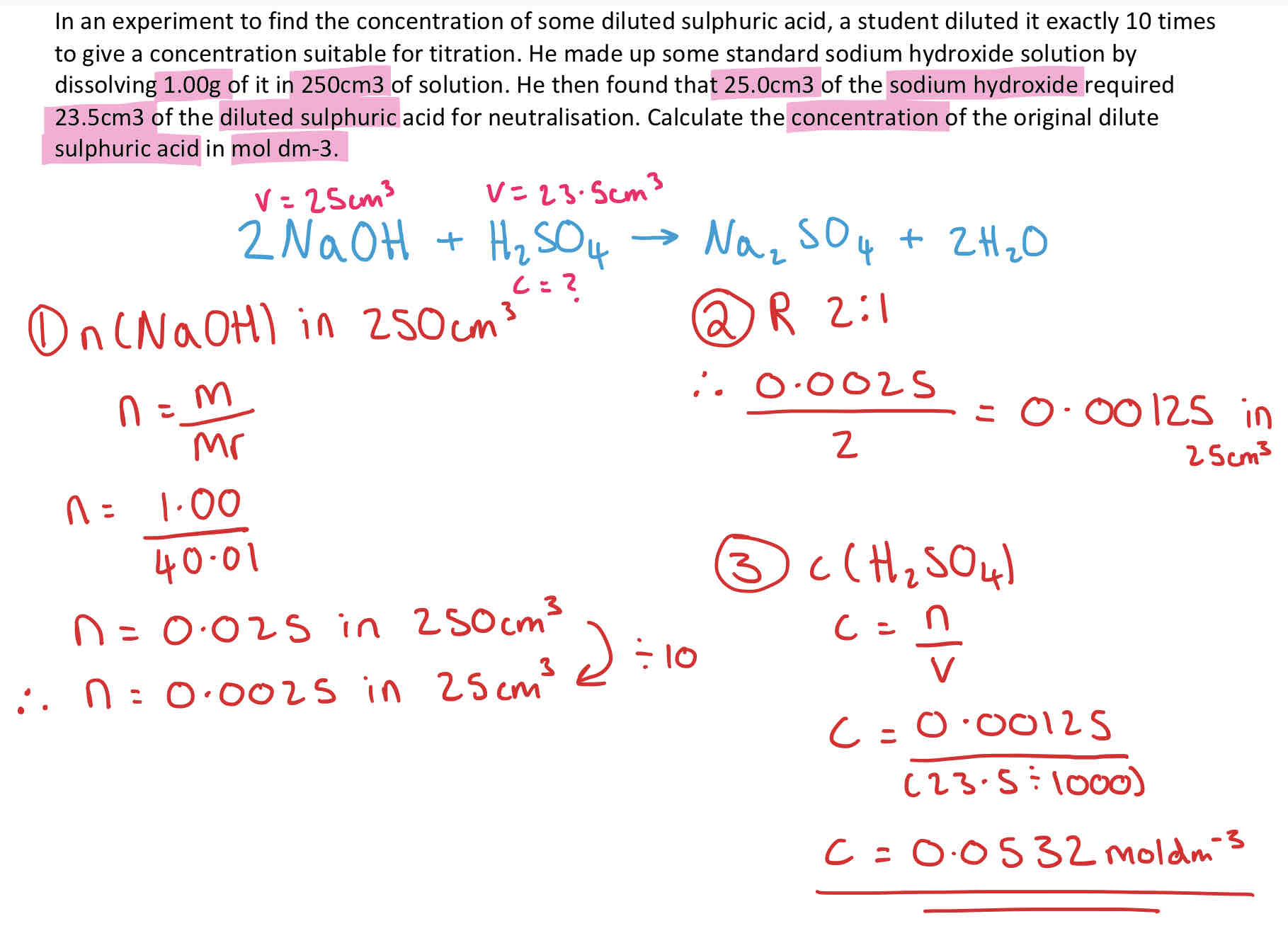
Calculation Involving Solutions - Concentration + Example
Concentration describes the amount of a substance dissolved in another substance. (Solutions)
no. of moles = volume (dm3) x concentration (mol dm-3)
Conversions:
1dm3 = 1 litre
1dm3 = 1000cm3

How do you convert a volume into mass for density?
1cm3 = 1g (stays the same)
How do you convert from gdm-3 to mol dm-3/ cm3 to dm-3? + Example
To turn from g dm-3 to mol dm-3 you Divide by Mr.
To turn from mol dm-3 to g dm-3 you Multiply by Mr.
To turn cm3 into dm-3 you Divide by 1000.

Tricker Concentration Calculation
Sources of error when doing titrations
Reading the burette/ pipette inaccurately
Must read from the bottom of the miniscus and at eye level to reduce error and over/ under filling the burette/ pipette
Difficult to see when Indicator changed colour/ therefore value of titre is more, since end point overshot
Human error due to colourblindness/ different levels of colour recognition.
How do we weigh solids?
Weigh:
Mass of weighing boat
Mass of weighing boat & solid
Mass of weighing boat & residue
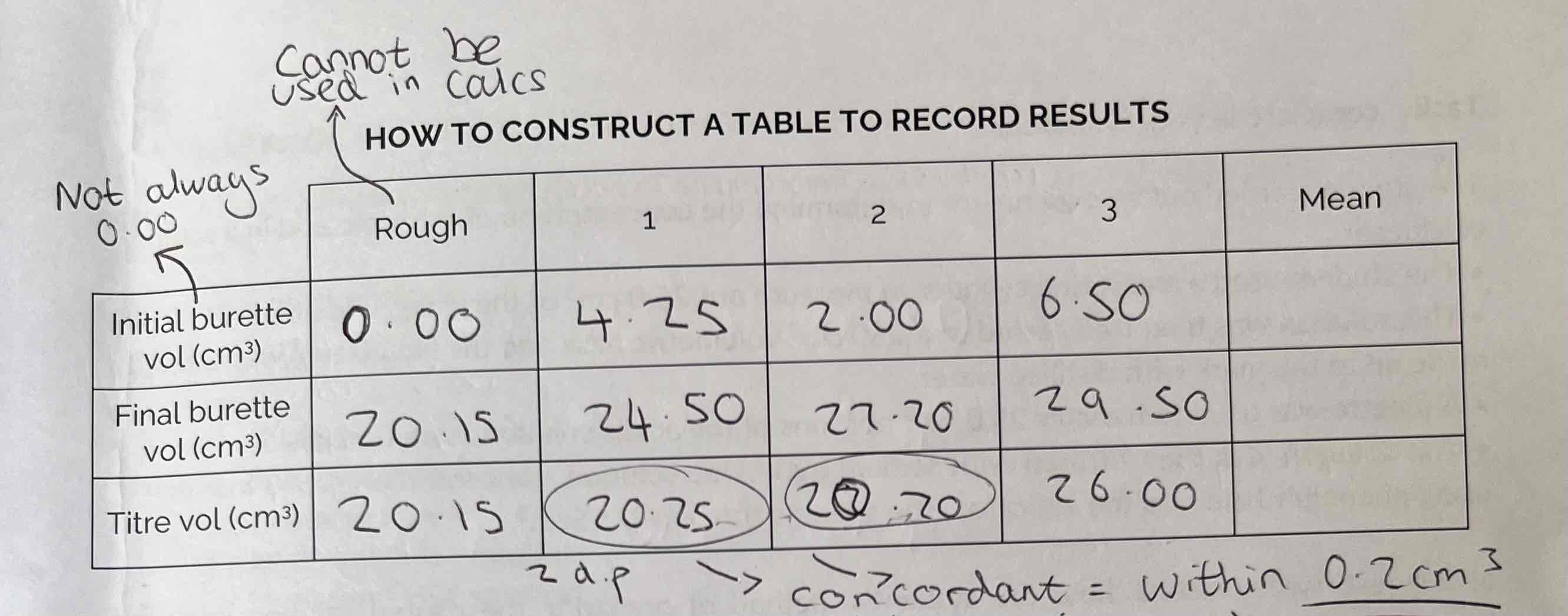
Results Table & Mean
Concordant results are within 0.2 cm3 (volume)
All titration values in a table should be to 2 decimal places
The mean should only be calculated from results that are concordant.
How do you know that the reaction has finished?
If the reaction produces a gas; when it stops fizzing.
Improvements to titration method
use larger beaker / conical flask - prevents acid spray from escaping (1)
Wash your equipment into the flask to ensure it all reacts - rinse beaker and add washings to flask - ensures no acid left in beaker
use more acid (e.g HCl) / less chalk, gives larger titres therefore smaller percentage error
crush the chalk / heat the solution / stir the solution, speeds up the reaction
Why do we rinse burette with Silver Nitrate?
To get rid of residue, as AgNO3 might react with residue, reducing its moles, therefore affecting titration volume.
Why can’t we use a solution (base) that is too concentrated?
The titration volume might be too large for neutralisation, and you would run out of solution (acid) in the burette if you don’t dilute solution (base).
How do you dilute seawater by a factor of 5?
Using a volumetric pipette, transfer 200cm3 of concentrated base solution (e.g seawater) to a 1000cm3 volumetric flask
Add deionised water up to the 1000cm3 mark, making sure the bottom of the meniscus sits at the mark
It would also work if we used 20cm3 of base solution in 100cm3 flask. As ratio is still 1:5
To dilute by a factor of 10 we can use 25cm3 sample in 250cm3 flask. Ratio is 1:10
Preparing a standard solution
Weigh the solid on a mass balance & record the mass - Tip solid into beaker & re-record boat mass.
Dissolve the solid in a small volume of deionised water. Tip into volumetric flask using a funnel.
Rinse the equipment - Rod, beaker & funnel x3
Standardise to 250cm3 using a dropper pipette, add deionised water up to the 250cm3 line (miniscus).
Shake well to mix (invert).
Suggest why you do not need to measure the volume of water accurately
it is the number of moles of ascorbic acid used that is important / concentration of acid is not important.
Why might someone heat the solution?
heated to ensure that all the acid / the e.g tablet dissolved
Carrying out a titration
Flush the burette with the substance we are filling it with, to remove impurities. Refill it with fresh substance to ensure moles is accurate. (Flush & fill the nozzle)
Use a volumetric pipette to measure exactly 25/ 20/ 50/ 10 (cm3). Transfer it into conical flask.
Rough Titration - Gives us a rough idea of titration volume needed for neutralisation. We can slow down before this point in repeats to improve accuracy. Slower rate of flow will increase accuracy as the end point can be seen more clearly.
Read value at eye level, from the bottom of the meniscus.
Repeat the titration - Refill burette (not necessarily to 0.00cm3). Flush conical with deionised water & refill using pipette.
Swirling the flask ensures even mixing of 2 solutions, so end point can be seen more accurately.
Reduced flow rate, drop by drop, to avoid overshooting the end point.
The white tile provides control, allows the colour change for end point to be seen more accurately.
Indicator allows the endpoint of the reaction to be seen more accurately.
What is % error + How do you reduce % error?
% error = no. of readings x 0.05/ titre (volume)
Use more concentrated stock solution of Sodium Carbonate.
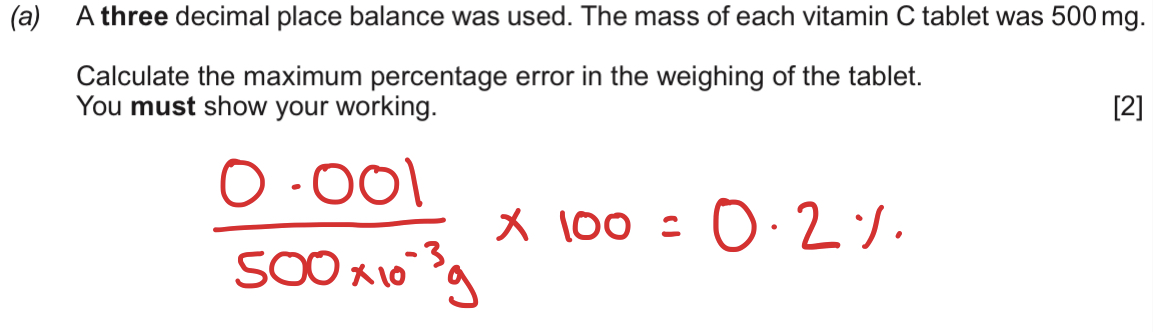
A student filters the solution before the reaction is complete. State what effect, if any, this would have on the value of the tire.
value of tire would increase (1)
fewer moles HCl react with limestone therefore more moles NaOH needed to react with excess (1)
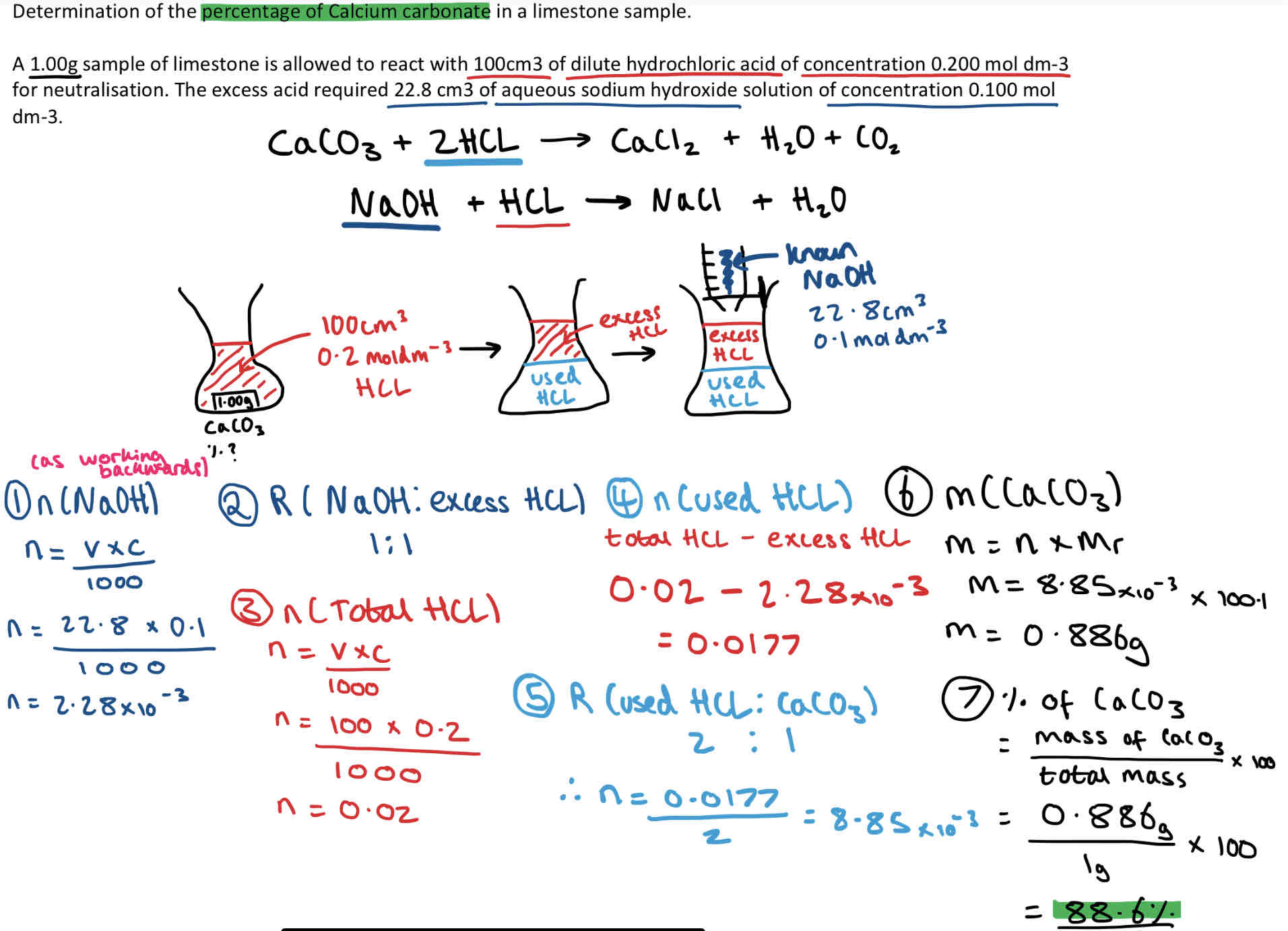
When do we use a back titration?
Sometimes it is not possible to use a standard titration method. This could be because:
The substance is thought to be impure so the molarity is unknown.
The base is an insoluble salt like copper oxide.
Instead we use a back titration (when solid reactant is insoluble).
Back Titration Calculation Example
Steps to work out:
moles of Sodium Hydroxide (titration solution) - n = m/ Mr
Ratio (NaoH: excess HCL)
moles of total HCL
moles of used HCL (total HCL - excess HCL)
Ratio (used HCL: CaCO3)
mass of CaCO3 - m = n x Mr
% of CaCO3 = mass of CaCO3/ total mass x 100
Double Titrations
A double titration is carried out when you have a mixture of bases in a sample solution.
This is usually a hydroxide and a carbonate as hydroxides react with COz in the air to form carbonates.
The goal of the practical is to find out the concentrations of the two bases in the original mixture.
Titre 1 - Phenolphthalein/ End Point 1

Titre 2 - Methyl orange

What does each stage relate to?
The first stage of the titration (phenolphthalein colour change) relates to the concentration of the hydroxide and carbonate.
The second stage (methyl orange colour change) relates to the concentration of the hydrogencarbonate (and therefore carbonate).
Double Titration Calculation Method - NaOH + Na2CO3
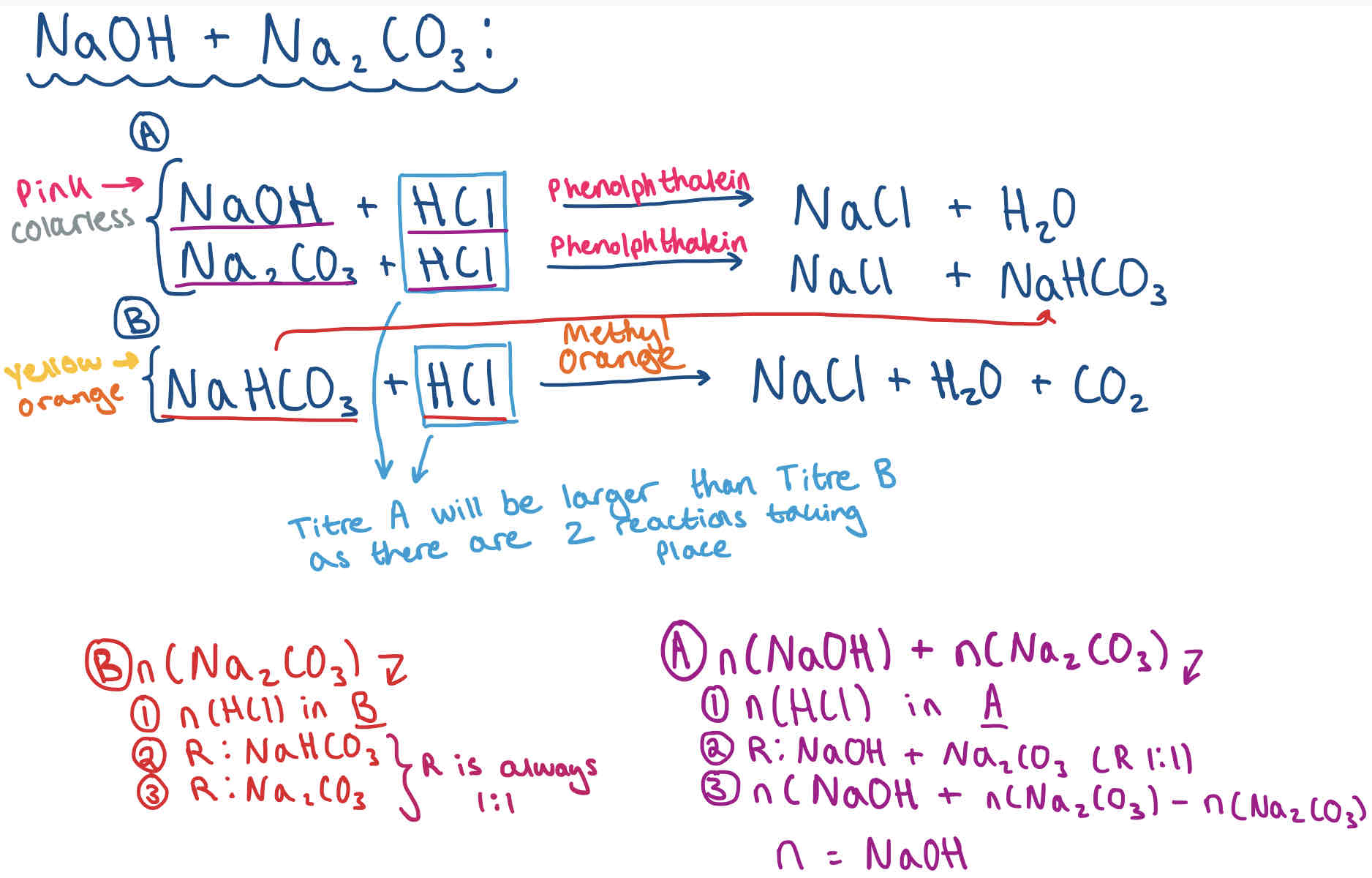
Double Titration Calculation Example
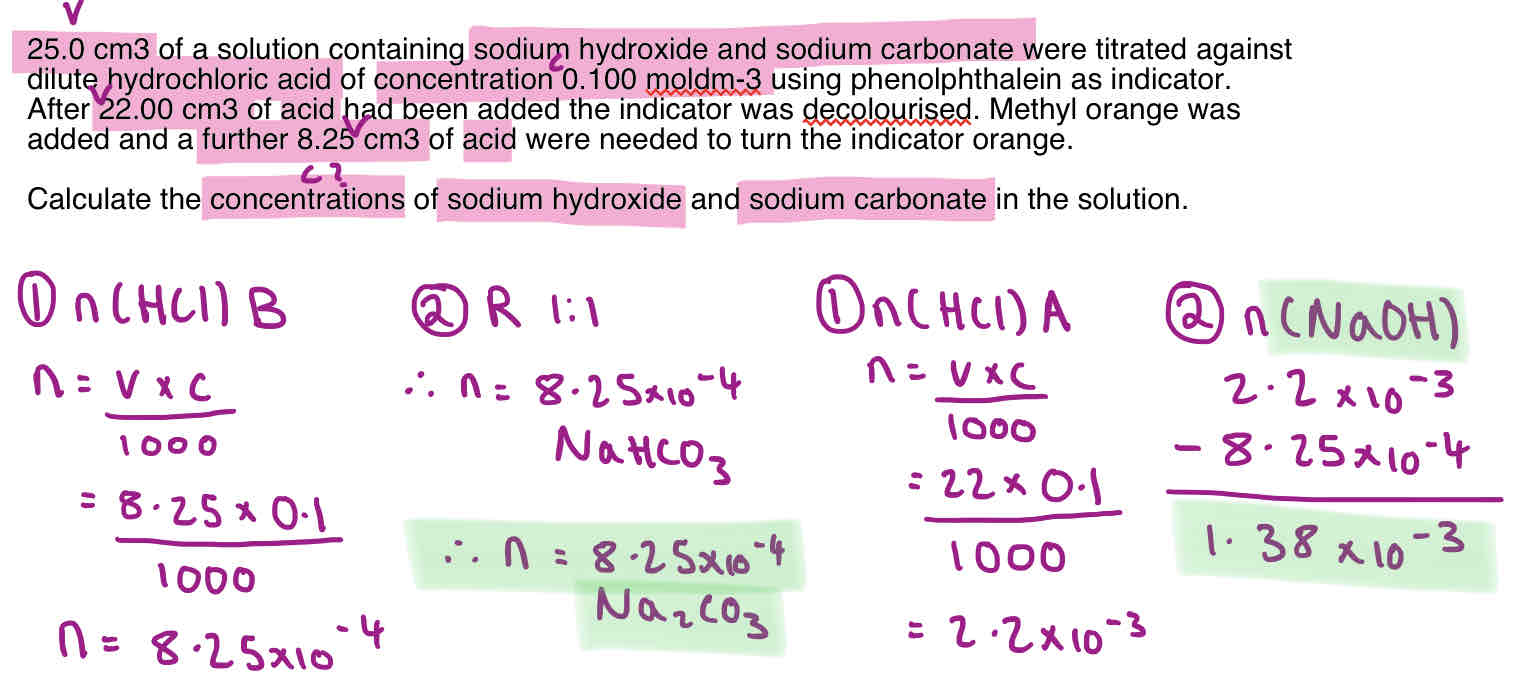
Double Titration Calculation Method - Na2CO3 + NaHCO3
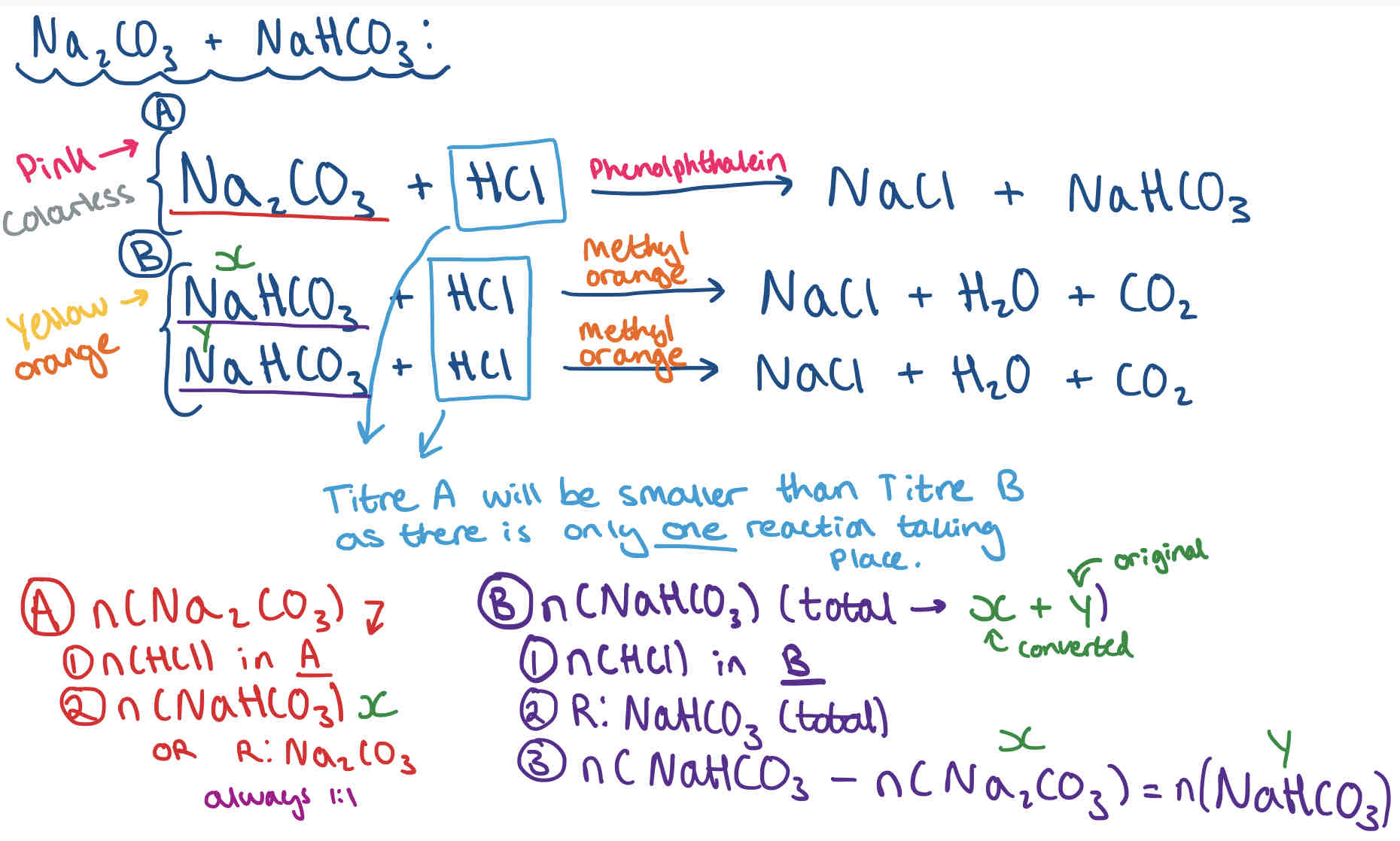
What is Avogadro’s Law?
Equal volumes of gases at the same temperature and pressure contain equal number of molecules.
e.g 20cm3 of H2 at 5oC/ 1 atm and 20cm3 of CO2 at 5oC/ 1 atm contains the same number of molecules. (under the same conditions, irrespective of the size of the molecules)
Avogadro’s law only applies to gases.
Some questions ask about the volume of air, not oxygen. Air is only 1/5 oxygen, so you need about 5 times more air than oxygen.
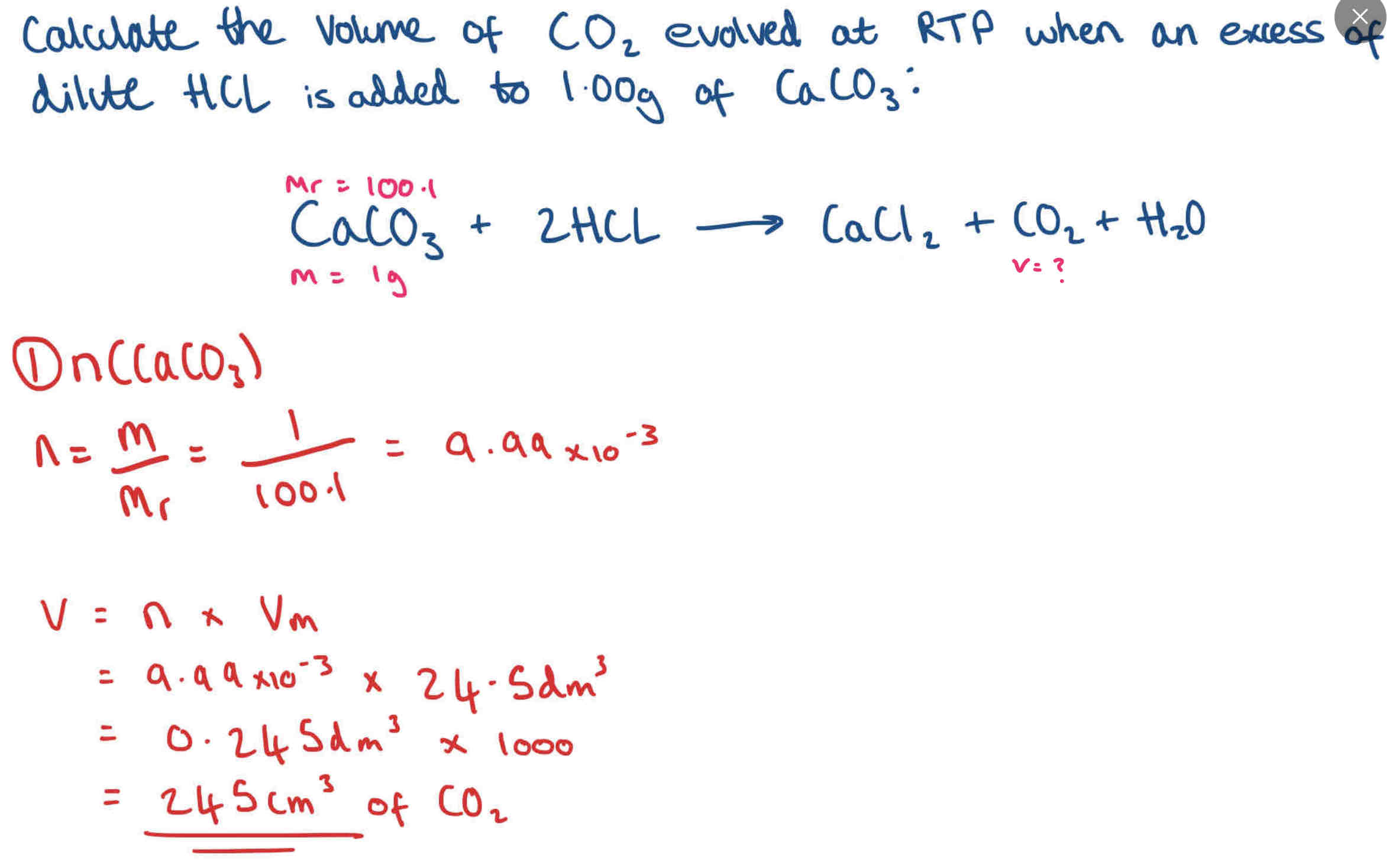
How do you calculate the molar volume of a gas?
At standard temperature and pressure (STP) it is apx. 0oC and the molar volume will be about 22.4dm3(22,400cm3)
At room temperature and pressure (RTP) it is apx. 25oC and the molar volume will be about 24.5dm3 (24,500cm3)
Particles have a higher energy at a higher temperature, so they take up more room (increases volume).
At STP and RTP the pressure is 1 atm (1.01 ×106 Pa)
Equation to work out moles of gases
moles = volume (gas)/ molar volume
n (mol) = v (dm3)/ Vm (dm3/mol OR dm3mol-1)
Gas Calculation Examples

Harder Gas Calculation Examples
Work out moles, then volume.
Ideal Gas Equation
pV = nRT
Where:
p is measured in pascals (Pa)
V in cubic metres (m3)
n in moles (mol)
T in kelvin (K) - (temperature in oC + 273 - given in data sheet)
R is 8.31Jmol-1K-1 (molar gas constant given in data sheet)
Conversion:
1m3 = 1,000dm3 = 1,000,000cm3
(x by 1,000) (x by 1,000)
Ideal Gas Calculation Example
In this example, we can’t use n = v/ Vm as pressure is not 1atm.
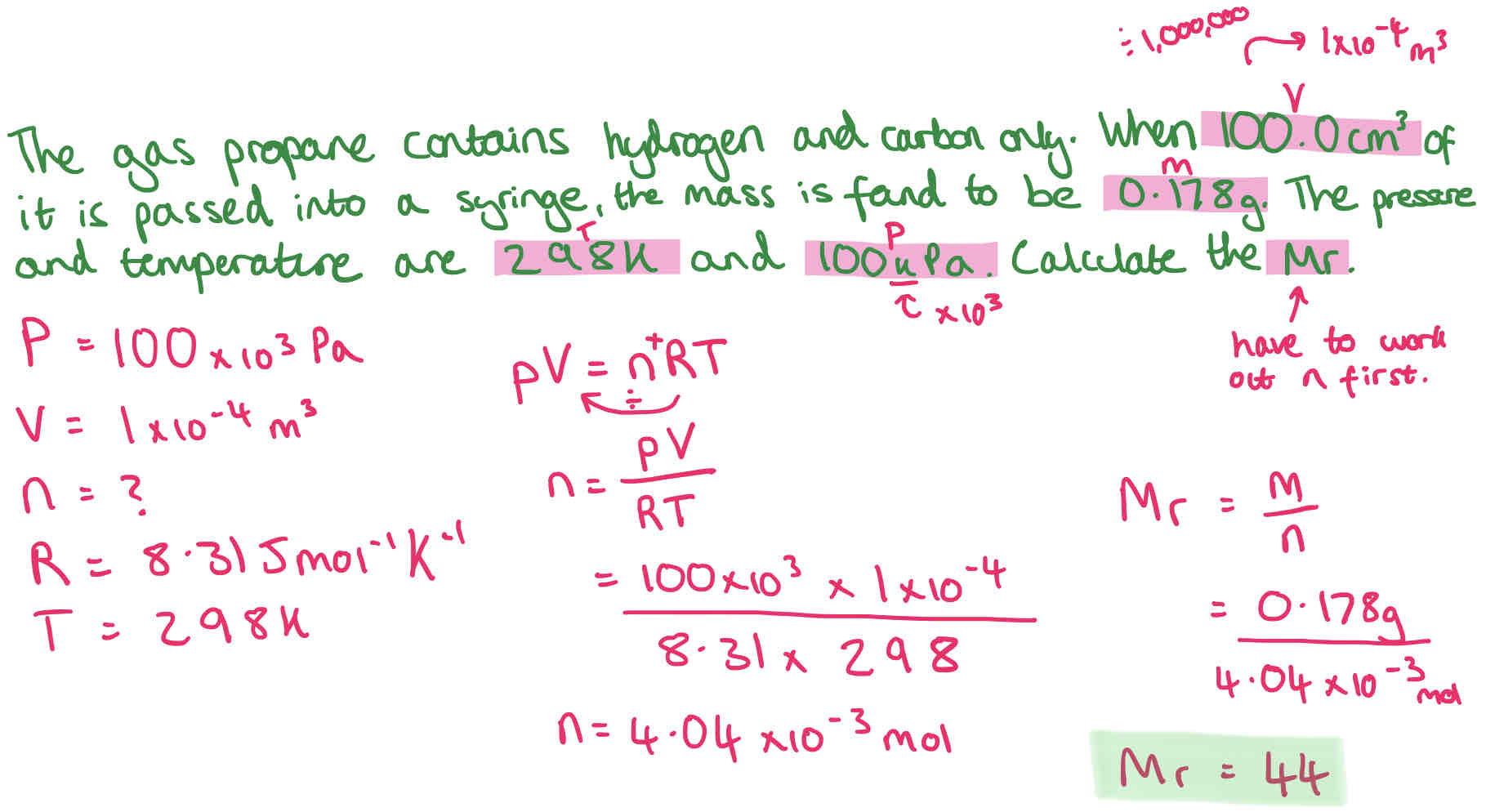
Gas Law Conversions - Boyles Law + Example
If temperature is constant, p1 x v1 = p2 x v2
Where:
1 represent the original conditions, and 2 the final situation is an enforced change of p1 or v1 is made.
P is in Pa
V is in m3
If temperature is not constant must divide each by T1 & T2
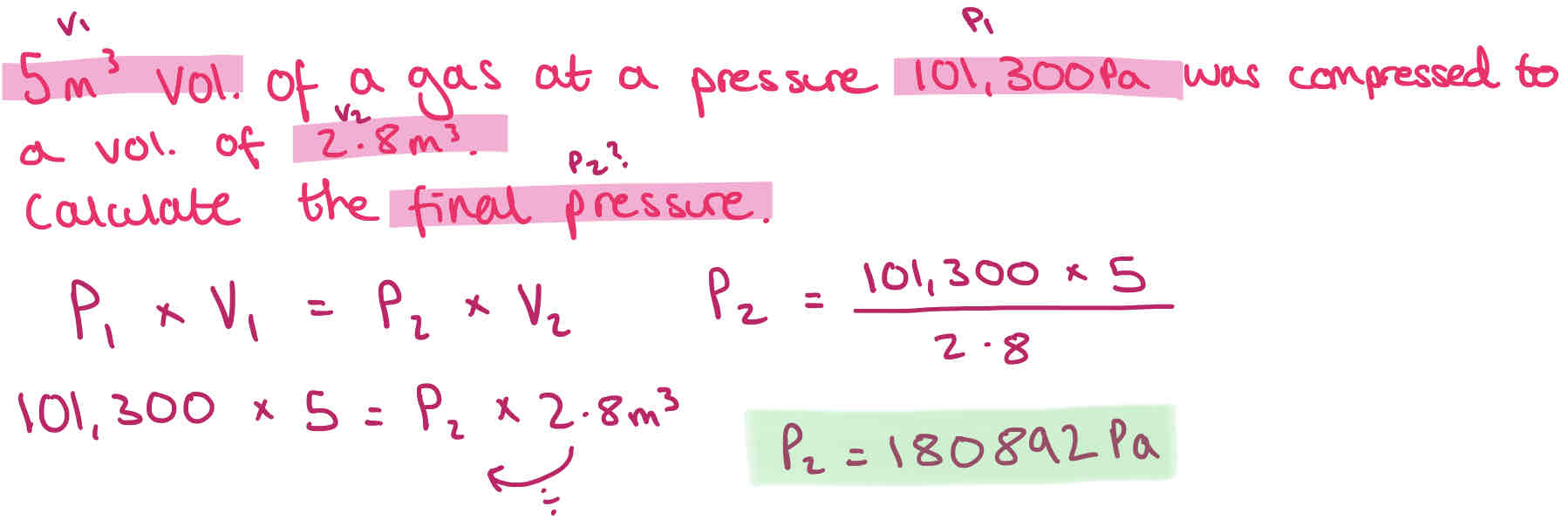
Limiting Reagents of Reactions - Method + Example
Work out moles of each reactant separately
Whichever reactant has lower mole is the limiting reactant/ reagent
Use the moles of the limiting reactant in any subsequent calculations

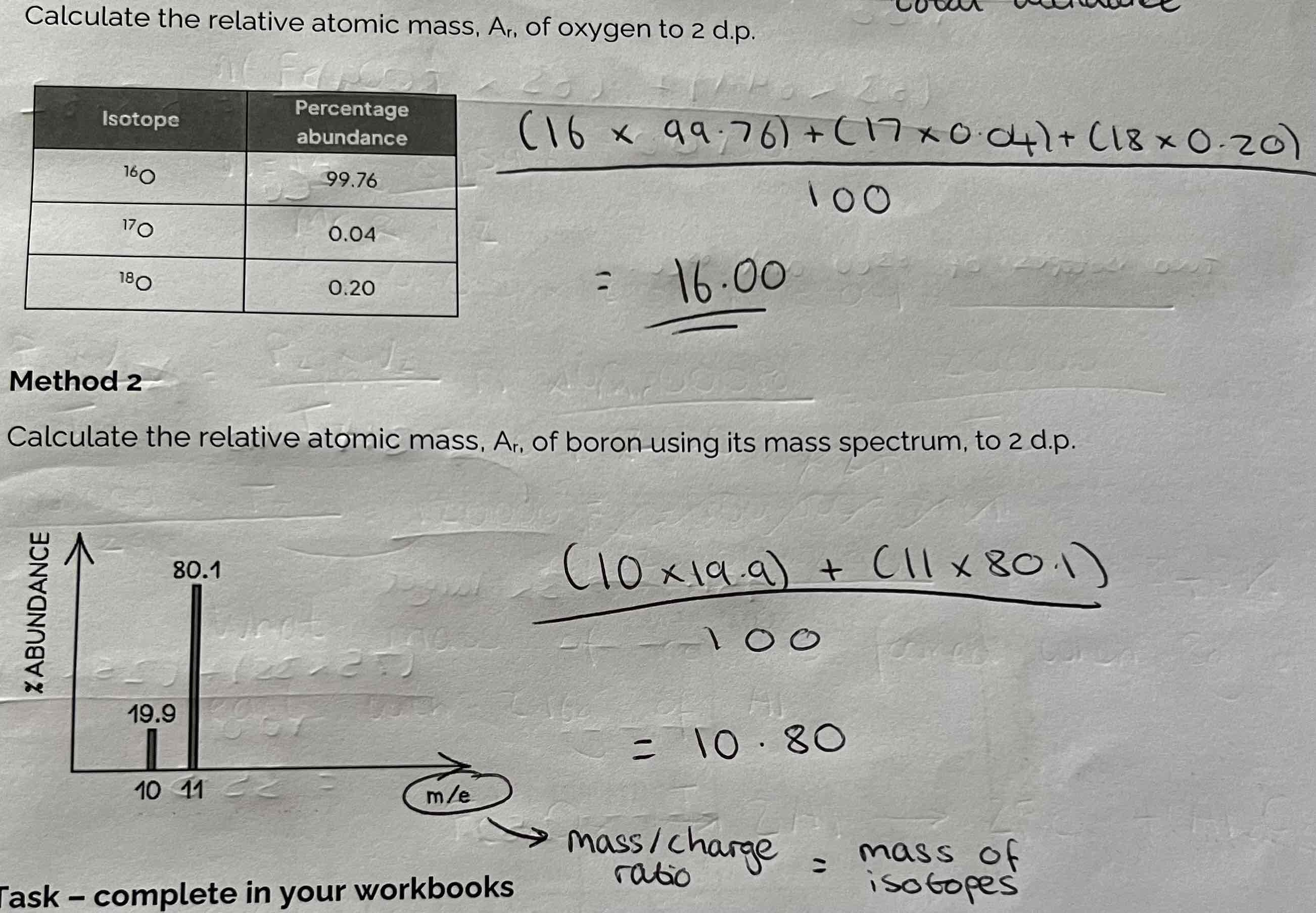
Using Mass Spectrometry to find the Ar of an element from its isotopic abundance + Examples
(Isotope 1 x Abundance 1) + (Isotope 2 x Abundance 2)/ Total abundance
The peaks, at m/z values show the different isotopes of an atom.
State how magnesium ions are formed in a mass spectrometer
(gaseous magnesium) atoms bombarded by electrons
State how magnesium ions are separated in a mass spectrometer
deflected through a magnetic field
Chlorine
Ratio for 1Cl Atom is always 3:1
Ratio for 2Cl Atoms is always 9:6:1
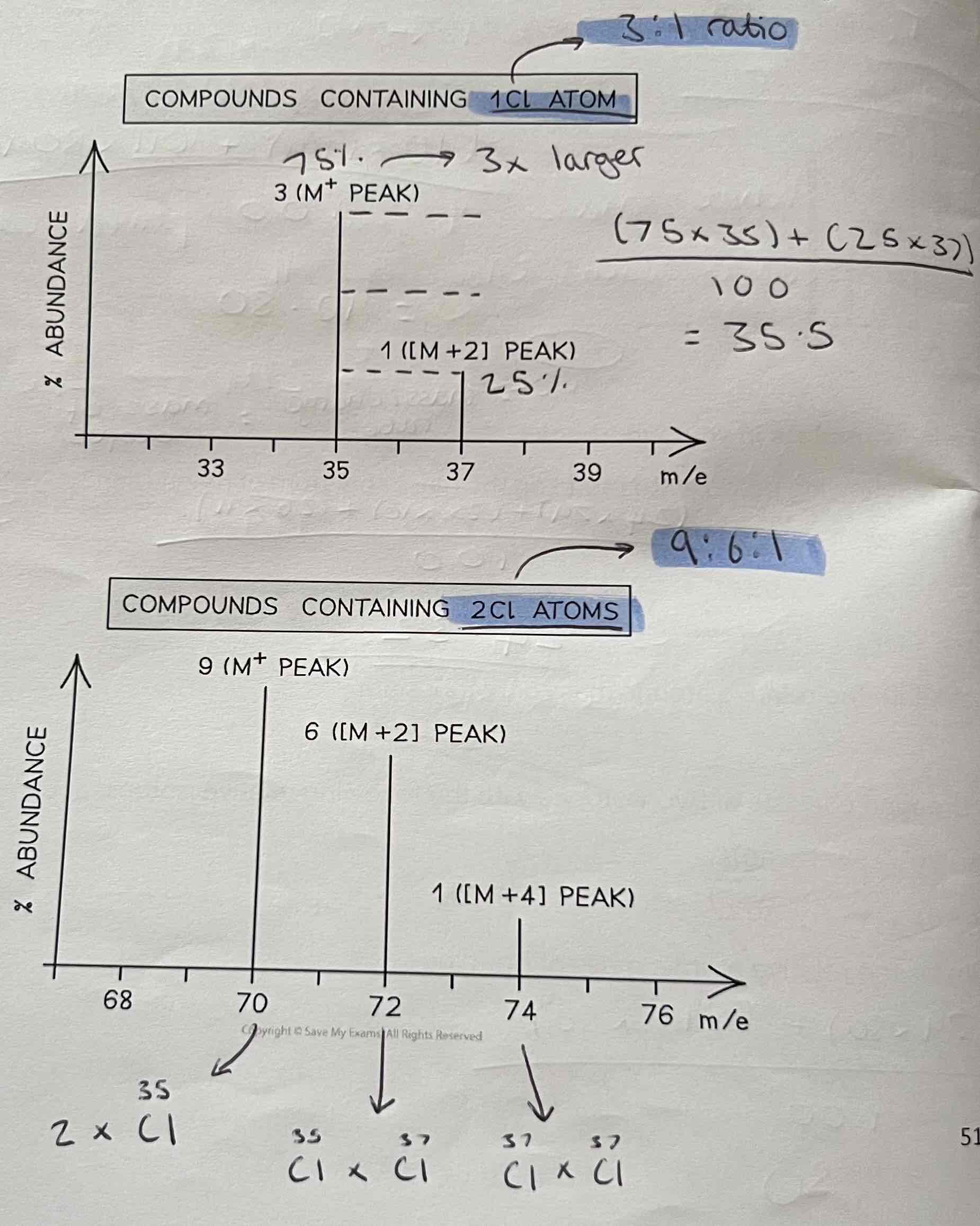
Example Question - m/z charge graph
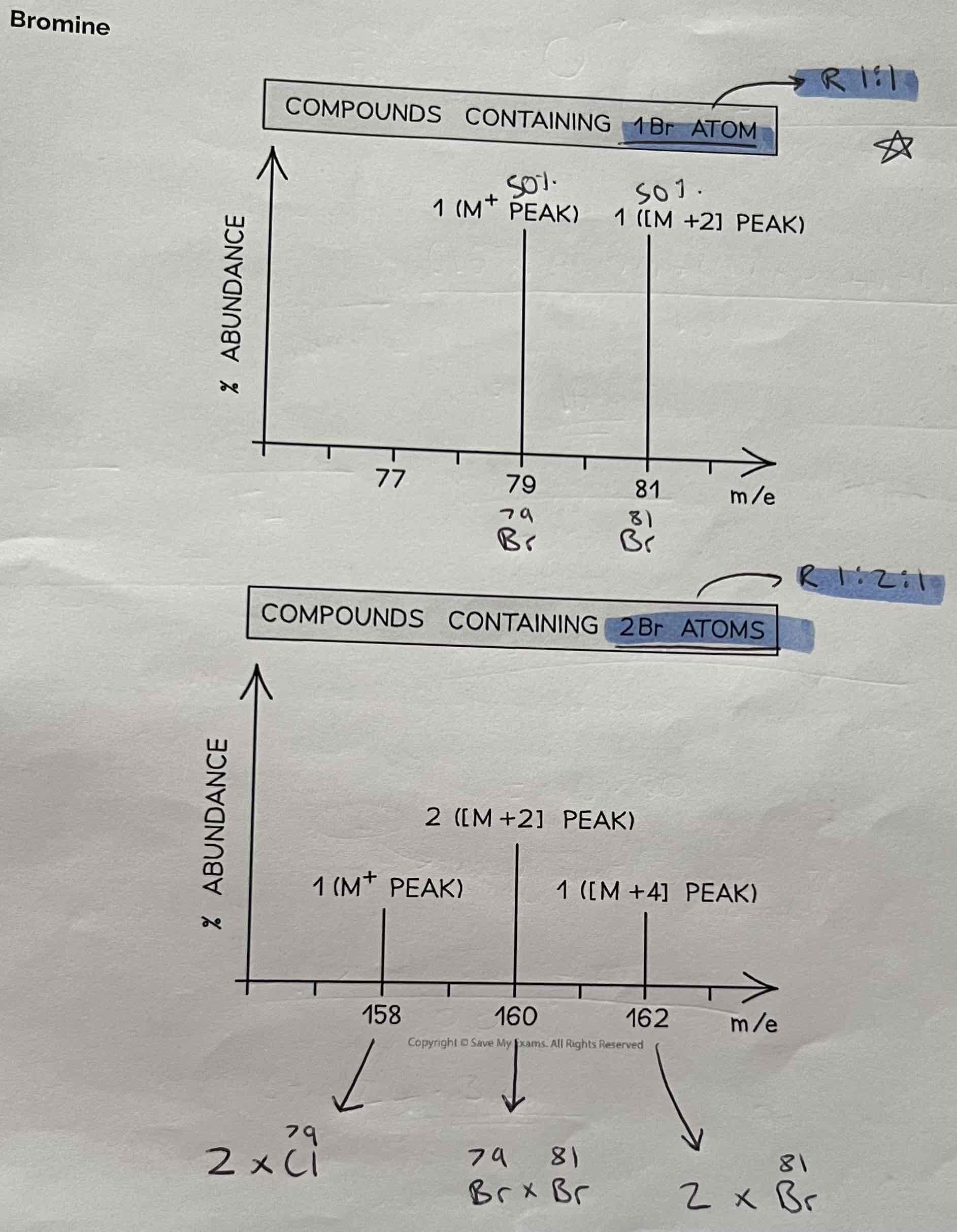
Bromine
Ratio for 1Br Atom is always 1:1
Ratio for 2Br Atoms is always 1:2:1