Animes and Forming Aliphatic / Aromatic Amines
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
What are amines?
A compound which has a nitrogen atom directly bonded to a carbon atom of an alkyl or aryl group.
What is a primary amine?
A compound where the nitrogen atom is bonded to only one carbon atom of an alkyl / aryl group.
What is a secondary amine?
A compound where the nitrogen atom is bonded to only 2 carbon atoms.
What is a tertiary amine?
A compound where the nitrogen atom is bonded to 3 carbon atoms.
What is a quaternary amine?
A compound where the nitrogen atom is bonded to 4 alkyl / aryl groups.
The nitrogen is positively charged; this is often referred to as the “quaternary ammonium ion.”
What is an aromatic amine?
A compound where the nitrogen atom is directly bonded to a benzene ring.
What two methods can be used to form aliphatic amines?
Reacting halogenoalkanes with excess ammonia.
Reducing nitriles.
Give the name of the reaction of a halogenoalkane with excess ammonia to form an amine.
Nucleophilic substitution.
The ammonia (NH3) acts as the nucleophile.
State the reagents and conditions needed to form amines by reacting halogenoalkanes with excess ammonia.
Reagents:
Excess ammonia dissolved in ethanol
Conditions:
Heated under reflux
Describe how amines are formed by reducing nitriles.
Amines can be formed by reduction of nitriles:
A suitable reducing agent (LiAlH4) dissolved in dry ether is used.
State the method used when forming aromatic amines (e.g. phenylamine).
Reduction of nitrobenzene.
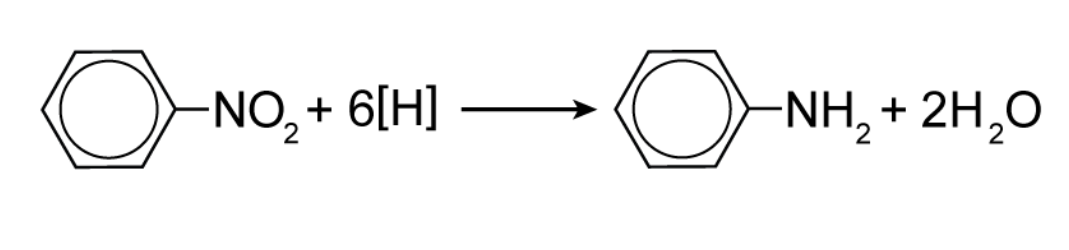
Describe how nitrobenzene can be reduced to form phenylamine.
Reagents:
Tin catalyst
Concentrated hydrochloric acid (HCl)
Sodium hydroxide (NaOH)
Conditions:
Heated under reflux
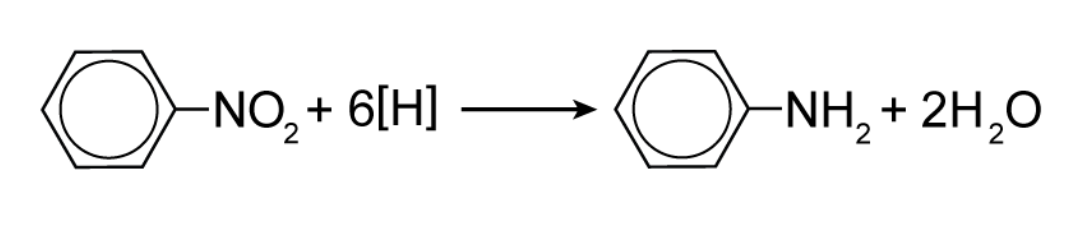
Why is sodium hydroxide (NaOH) added when reducing nitrobenzene to form phenylamine?
When a tin catalyst and concentrated HCl is added to nitrobenzene, C6H5NH3+Cl- forms.
NaOH is added to decompose the salt into phenylamine together with a complicated mixture of tin compounds.
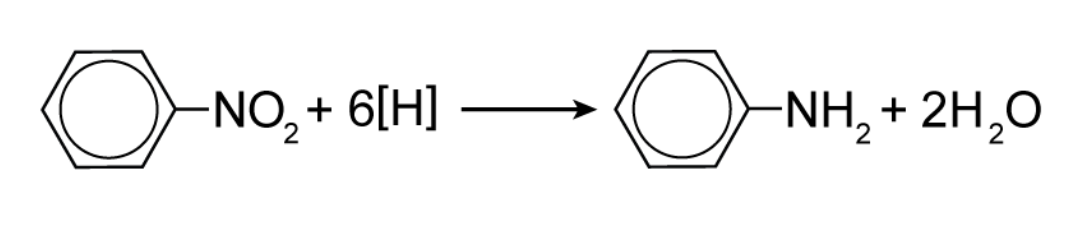
How is phenylamine separated when reducing nitrobenzene?
Fractional distillation / steam distillation