bio scientific inquiry unit 1
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
80 Terms
base unit for length
meter (m)
mm, cm, m, km
what is used to measure length
ruler
base unit for mass
grams (g)
mg, g, kg
what is used to measure mass
triple beam balance or electronic scale
base unit for volume
liter
ml, l
what is used to measure volume
graduated cylinder, beaker, flask
base unit for temperature
celsius
degrees C
what is used to measure temperature
thermometer
what is the symbol for kilo
k / x 10³
what is the prefix of 1/1000 of something
mili
what is 1/100 of a meter
cm
what are two symbols that are used to measure volume
ml, l
volume
amount of space object takes up
mass
amount of matter object takes up
temperature
molecular movement in substance
length
distance from 1 point to another
what is the term to know the metric conversion
king henry died by drinking chocolate milk
what is the dimensional analysis formula if you are moving the decimal to the right
ex: 1L to mL
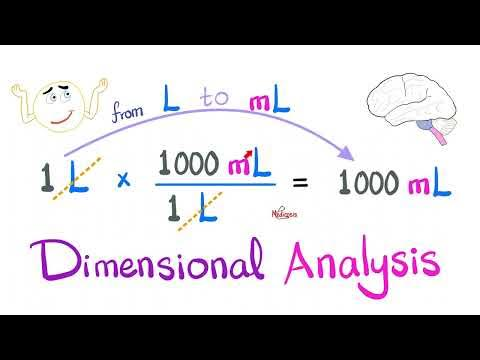
what is the dimensional analysis formula if you are moving the decimal to the left
ex: 1000mL to L
1000mL /1 x 1L / 1000mL
what do you always do if there is a digit not recorded on the equipment
estimate last digit/no naked numbers
ex: 3.0
what do you always do before using the tripe beam balance
0 the balance
what should you do if glassware is broken
report to the teacher immediately
what do you do when you are handling glassware
put on safety goggles
what should you always do before storing a flask
close it with a rubber stopper
what do you use the flask for
to stir and swirl the liquid inside
what is a beaker for
measuring and pouring liquids
what is a graduated cylinder used to measure for
precise volume of liquid
how should you measure liquid from a graduated cylinder
at eye level (below the curve) from the bottom and always estimate last digit
what is a meniscus
curved surface of liquid in graduated cylinder
what are test tubes used for
containing and mixing liquids
what can a test tube be closed with
rubber stopper
how is a test tube heated
into a beaker of water that is on a hot plate (hot water bath)
what are hot plates used for
heat liquids inside of test tubes, flasks, and beakers
what are the safety rules when using a hot plate
goggles should be worn when using heat
never heat a CLOSED container
never heat an object facing you or others (should always be facing away while heating)
what is temperature measured in
degrees celsius
what is the freezing temp for water
0 c
what is the boiling temp of water
100 c
what is normal human body temp
37 c
what is room temp
23 c
what is proper lab attire
tight clothing
hair tied up
no open toe shoes
what items are never allowed in a lab
food, drinks, and gum
what conditions should lab areas be kept in
clean and no unnecessary materials
what 3 times should you wear safety goggles
working with glassware
working with chemicals]
heating stuff
what are 2 rules when heating a test tube
point away from you and others
use tongs to pick it up
why should a closed container never be heated
the gas inside can expand and explode
What might be on glassware that is “dirty?”
Leftover chemicals
Explain the proper way to “smell” a chemical.
Use a wafting motion
When doing an experiment, what should be done with chemical wastes?
dispose of materials as instructed, never put materials down the sink or in the trash without permission
why is it important to throw away glassware in the glass bin
So the people throwing it away after are aware that they are handling with glass and don't get cuts or hurt
Identify 5 pieces of lab safety equipment that you need to know the location of in the classroom
fire extinguisher, fire blanket, first exit, safety shower, and eyewash station
When you complete your lab, what should you do
notify teacher and follow cleanup instructions and return all equipment to its proper place
what should you do when someone is on fire
use a fire blanket because fire extinguishers have a lot of chemicals
what is quantitative data
data that includes numbers
what is qualitative data
data that doesn’t include numbers like characteristics or stuff from the five senses
what is the independent variable
the variable that you control on purpose
what is the dependent variable
the variable that is affected by the iv and measured
what is the format of a hypothesis
if (iv), then (dv)
what is a hypothesis
predicts the outcome of an experiment and based on background knowledge
what is a control group
the group not being tested on
what is the experimental group
the group being experimented on
how do you increase the validity of results
increasing sample size and doing multiple trials
what are constants
things that stay the same in a experiment like the type of something or the equipment
how do you make a valid claim
by using evidence like quantitative or qualitative data
what does TAILS stand for in graphing
t = title
a = axis
i = intervals (are they even)
l = legend/key
s = scale
how much does the data have to take up in a graph
about 75%
do you have to start at 0 for a graph
no unless its a bar graph or in the data set
what could the title of a graph be
effect of iv on dv and include units
what do you include when labelling the y and x axis
the units
when making a key what do you put
the value of each box
never connect to 0 unless
its a data point
what is a line graph
tracks change over intervals like temperature, pH, time, etc
what is a bar graph
categorical data and compares data between groups
what is a histogram
shows distribution
what is the difference between a histogram and bar graph
histogram bars are touching and range of data is divided into equal intervals
what is a positive relationship
the data increases
what is a negative relationship
the data decreases
what is a direct relationship and what other relationship is it
increases and linear
what is a indirect relationship and what other relationship is it
decreases and negative
what is a non-linear relationship
exponential or fluctuating
what do you put around the unit in a graph
parenthesis
ex: (kg)