N412 MT #2 ALL
1/248
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
249 Terms
PAD vs PVD
Both are progressive diseases that narrow or block blood vessels, however, there are differences.
Peripheral vascular disease (PVD):
PVD is a broader term that includes any blood vessel encompassing arteries, veins and lymphatic vessels.
PVD doesn’t cause structural damage of the vessel.
Peripheral arterial disease (PAD):
Affects arteries alone and damages tissues of vessel walls.
Tissue damage is caused by accumulation of fat.
Peripheral Arterial Disease (PAD)
Condition in which thickening of artery walls leads to progressive narrowing of arteries of the upper and lower extremities.
Most arterial vascular disease is a result of atherosclerosis (PAD is a marker of advanced atherosclerosis).
Arterial disease may occur suddenly, following an embolus, or thrombus, or insidiously as in atherosclerosis.
When symptomatic ⇨ intermittent claudication (exertional related) – classic Sx
With progression of disease, symptoms may occur at rest, often in toes, and worse at night
Clinical symptoms occur when vessels are 60-70% occluded.
Physical exam: smooth, shiny, hairless skin in lower extremities; ↓ or delayed distal pulses
PAD - Diagnosis
For assessing blood flow & outline the vascular system:
Health hx & physical exam
Angiography
Ankle-brachial Index (ABI)
Doppler ultrasound studies
Ankle-brachial Index (ABI)
ABI is a peripheral artery disease screening tool
Normal persons:
SBP in legs slightly higher than in arms (ankle-brachial index > 1.0)
People with PVD:
ABI decreases (< 1.0)
Especially before & after exercise
Calculation of ankle-brachial index (ABI)
ABI = highest systolic ankle pressure (mmHg) / highest systolic arm pressure (mmHg)
Normal ABI = > 1.0
ABI for pts with claudication = 0.5 - 0.9
ABI for pts with rest pain & critical leg ischemia = < 0.5
Lower the ABI, the greater the arterial impairment
Acute Arterial Ischemia (AAI) - ??? & 6Ps
Sudden interruption of blood flow to tissue, organ, or extremity that, if left untreated, would result in tissue death
Caused by embolism, thrombosis of a pre-existing atherosclerotic artery, or trauma
A thrombus from heart is most frequent cause of acute arterial occlusion
Thrombi that originate in (L) side of heart → majority obstruct an artery of lower extremities (e.g., iliofemoral, popliteal, tibial)
Six Ps of acute arterial ischemia:
pain, pallor, pulselessness, paresthesia, poikilothermia (coldness), and paralysis
Management of Peripheral Arterial Disease
Risk factor modification
e.g., Diabetes, smoking, cholesterol, HTN
Drug therapy
Antiplatelet agents, e.g., ASA (ASA + Plavix together NOT recommended)
Pentoxifylline (Trental) – for intermittent claudication
Exercise: cessation of smoking combined with supervised exercise
Nutritional therapy: to ↓ BMI
Complementary & alternative therapy: vitamin, mineral, herb supplements → but current research data insufficient
Critical Limb Ischemia
Characterized by chronic ischemic rest pain lasting > 2 wks, arterial leg ulcers, or gangrene of the leg as a result of PAD (peripheral arterial disease)
Optimal therapy is:
Endovascular procedure
Surgery
Reason for surgical procedure: for revascularization & to ↓ CVD event
PVD Endovascular Procedures (Interventional Radiological Catheter-Based)
Percutaneous Transluminal Angioplasty (PTA)
To restore blood flow with the use of a balloon-mounted catheter, the tip is advanced to where the stenosis is and inflated
PTA is an established treatment for peripheral arterial occlusion. For short stenosis or occlusion of iliac, femoral and popliteal arteries.
Stents
Deployment of expandable metallic devices within the artery immediately after PTA
To treat peripheral artery dissection (tear inner arterial wall)
Insertion of expandable metal stents to prevent restenosis.
Atherectomy
Removal of obstructing plaque with a high-speed cutting disc built into catheter end
Uses high speed revolving cutters to cut & remove an obstructing thrombus.
Cryoplasty
2 procedures: balloon angioplasty + cold therapy
The specialized balloon inflated with nitrous oxide that changes from liquid to gas as it enters the balloon & ↓ temp of gas to -10 ºC. The cold temp minimizes restenosis
Types of Reconstructive Surgery (5)
Aorto-Bifemoral Graft
Bypass extends from distal aorta to common femoral arteries.
For stenosis of aorta or iliac vessels.
Goes above the blocked/diseased section and connect it, then the blocked section is removed
Other ends of the graft are attached to common femoral arteries
Femoro-Popliteal Bypass
For occlusion in superficial femoral artery.
Graft is either a healthy blood vessel or a man-made material.
Femoro-Distal Bypass
For stenosis in distal vessels. Preferred to use saphenous vein in severe critical ischemia.
Graft extends from femoral to either peroneal or tibial artery.
Patency rate poorer than femoro-popliteal graft
Axillo-Bifemoral Graft
For aorto-iliac stenosis.
Restores blood flow to the legs by creating a pathway around blocked or narrowed aortic/iliac arteries
Done in severe PAD and uses a synthetic graft to connect axillary artery to femoral arteries
Femoro-Femoral Crossover Graft
For iliac artery occlusion.
Reroutes blood from the healthy femoral artery in one leg to the common femoral artery in the other leg
Immediate Post-Op Care Following Peripheral Bypass Surgery
Primary responsibility → early recognition of complications
ABC
VS - Per protocol (e.g.,. q15 min, q30min, & q1hr)
√ peripheral pulses : dorsalis pedis, posterior tibial using a doppler
Mark the location
Loss of pulses, or sudden ↑ of pain should be reported immediately
Observe CWMS: report sudden changes
Use a bed-cradle to aid observation, and protect heels
Observe wound for bleeding or hematoma formation
ABI measurements may be ordered.
Sudden ↑ in output in drainage tube → rupture of graft anastomosis
Any indication of graft occlusion or rupture → surgical emergency
Continuing Post-Op Care Following Peripheral Bypass Surgery
Analgesia for pain (i.e., epidural, or a PCA ) → for 24-48hs or until can take them orally
May need aggressive pain control d/t high tolerance to opioids
Preventative Abx – graft infection
Monitor U/O – how many ml/hr should it be? 30mls/hr
IV fluids via central line (i.e. CVP line)
Sliding scale insulin for diabetic patients
O2 as prescribed
Possibility of paralytic ileus in pts with aortic grafts → TX supportive care, IV fluids, electrolytes, ambulation in severe cases bowel rest with NG suctioning.
Sit upright, DB & C, gentle leg exercise to prevent chest infection and DVT
Mobility encouraged 1-2 days post-op
Elevate legs to prevent occlusion of grafts behind knee (i.e. femoral to below-knee popliteal graft)
SC Heparin: Why? Decrease risk of DVT
Anti-embolic stockings ⇒ not unless instructed by the surgeon - not recommended for ABI < 0.7
Inspect wounds for signs of inflammation (infection) → can lead to graft loss or sepsis
Sutures removed 12-14 days post-op
Drainage tubes
Signs of a Graft Occlusion
Think of symptoms d/t a blocked artery
Sudden loss of pulse in the extremity where the graft to place
Increased pain
Coolness to touch
Pallor or cyanosis Why?
Delayed cap refill in affected limb
Weakness or paralysis
Numbness or tingling (can be nerve compression)
Aortic Aneurysms
A permanent, localized outpouching, or dilation of vessel wall (congenital or acquired).
May involve aortic arch, thoracic aorta, abdominal aorta, or a combination.
Most are abdominal aorta below renal arteries.
75% occur in abdomen; 25% in thoracic aorta.
Dilated aortic wall becomes lined with thrombi.
Primary cause:
Degenerative
Congenital
Mechanical
Inflammatory
Infectious
Aortic Aneurysms - Classification
Aneurysms are classified as TRUE or FALSE
TRUE A: the wall of the artery forms the aneurysm, and at least one vessel layer is intact
True aneurysms subdivide into:
Fulsiform (bulges on both sides) aneurysm OR
Saccular (only bulges on one side) aneurysm → often seen in brain and more prone to rupture
FALSE A: (aka pseudoaneurysm)
not an aneurysm but a disruption of the layers of arterial wall → results in bleeding that is contained (blood pools in the injured area)
may result from trauma or infection, or at the site of peripheral artery bypass surgery
Aortic Aneurysms - Clinical Manifestations
Thoracic aneurysm - Often asymptomatic but can cause: ⇨ deep diffuse chest pain extending to interscapular area (shoulders)
Ascending aorta & aortic arch aneurysm:
hoarseness/SOB/cough d/t pressure on laryngeal nerve
angina from decreased blood flow to coronary arteries (increase risk of TIA d/t decreased blood flow to carotid arteries)
If aneurysm pressure on superior vena cava = JVD, edema to face and arms
Abdominal A A (triple A) ⇨ often asymptomatic, detected on routine physical exam
(= pulsatile mass in periumbilical area slightly left of the midline)
Lower back pain caused by compression on lumbar nerve
Pain that spreads to lower buttocks, groin or legs.
Epigastric discomfort
Aortic Aneurysms - Complications
Most serious → rupture of aneurysm
Rupture of a triple A signs
Flank ecchymosis (Grey Turner’s sign) - bruising on both sides of flank area
Indicates bleeding in the retroperitoneum space
Pancreatitis
Severe, sudden pain in abd, back, or groin
SOB, N/V, sweating, clammy skin, increase HR, dizziness
If ruptured blood leaks into thoracic or abdominal cavity, 90% mortality from hemorrhage
Aortic Aneurysms - Treatment
Goal: to prevent aneurysm rupture and extension of dissection
Conservative therapy is for…
Small, asymptomatic AAAs (4.0 - 5.5cm)
Size of aneurysm will determine the risk of rupture
Conservative tx:
Quitting smoking
Treating HTN w/ meds to keep it controlled
Regular U/S surveillance with referral to sx if aneurysm grows >5cm
Higher risk in females
Surgery is for…
Rapid expanding aneurysm (> 1 cm diameter ↑/year)
When pt becomes symptomatic
High risk of rupture (larger = higher risk of rupture)
Involves replacing abdominal aneurysm with a synthetic tube graft
Aortic Aneurysms - Surgery
Elective
During pre-op:
Hydration
Correction of electrolytes, coagulation, hematocrit abnormalities
Low Hct = significantly higher blood loss and needed fluid/blood replacements
Bowel prep
Procedure:
Incision of diseased aortic segment
Removal of thrombus or plaque
deployment & suturing of synthetic graft
Most resections done in 30 – 45 min
Requires cross-clamp clamping distal to aneurysm clamps are removed after the surgery.
Emergency
Ruptured aneurysm ⇨ 100% fatality without emergency surgery
Only minimal physical preparation is possible
Reassurance and emotional support for pt and family
Lethal complication in repair of ruptured AAA = intra-abdominal hypertension with associated compartmental syndrome
Reduces blood flow to organs → multi-system organ failure
AAA Post-Op Care
Typically admits to ICU post-op for 24 - 48hrs
ECG, Endotracheal tube, arterial line, CVP or Pulmonary artery catheter, peripheral IVs, foley catheter, chest tubes ( if thorax opened in surgery), possibly an N/G
Pain meds: either epidural catheter or PCA
Maintain adequate respiratory function, fluid & electrolyte balance
Assess graft patency
Monitor for infection
Monitor renal perfusion
Endoleak
Leakage of blood back into the old aneurysm
Most common complication
TX coil embolization
AAA Post-Op - Cardiovascular
Continuous ECG monitoring
Myocardial ischemia or MI during peri-op due to ↓myocardial O2 supply or ↑ demand
Dysrhythmia R/T electrolyte imbalance, hypoxemia, hypothermia, or myocardial ischemia
Electrolyte and ABG monitoring
Admin O2
Adequate pain control
Resume cardiac meds
AAA Post-Op - CNS
Assess LOC
Glasgow coma scale
With involvement of descending aorta, neurovascular assessment of lower extremities is important
AAA Post-Op - Peripheral Perfusion Status
Check all peripheral pulses q1h for several hrs (or per protocol) and then per routine
If surgery involves ascending aorta & aortic arch → emphasis is to assess carotid, radial and temporal pulses
If surgery involves descending aorta → assess femoral, popliteal, posterior tibial and dorsalis pedis pulses
Mark pulse locations with a felt-tip pen
AAA Post-Op - GI
After abdominal aortic surgery, paralytic ileus is possible D/T anaesthesia & manipulation of bowel (rarely lasts beyone POD4)
Intestines become swollen & bruised, paused peristalsis
NG tube ⇨ decompress and reduce aspiration of stomach contents
Return of bowel function. How would you know? → want early ambulation, BSx4, passing flatus
AAA Post-Op - Infection
Vascular graft infection
Admin broad spectrum antibiotic per order
Assess surgical site for infection
Examine all IV sites
Foley cath insertion site (removed ASAP)
Redness, swelling, warmth, discharge
Temp, regular labs (WBC), know S+S of sepsis
AAA Post-Op - Graft Patency
Adequate BP important: prolonged hypotension results in graft failure
How would you determine adequate blood flow? Look at the CWMS extremities below graft, urine output, MAP>60
Severe hypertension may cause undue stress on anastomosis sites
Why is this an important issue? Can cause leaking of blood or rupture of sutures
AAA Post-Op - Renal
Foley cath
Immediate post-op: record U/O q1h — maintain 0.5–1 ml/kg/hr
I & O, daily weight until resumes regular diet
Monitor lab work for renal function. Which ones? eGFR, BUN, Cr
Factors for ↓ renal perfusion
Embolization to renal artery(ies)
Individuals at high risk for renal failure include patients with hypotension, prolonged clamping during surgery, preexisting renal disease or diabetes.
Aortic Dissection
Often misnamed “dissecting aneurysm”, but is NOT a type of aneurysm
Dissection results from creation of a false lumen
Is a tear in aortic intima through which blood enters and creates a false lumen between intima and media of blood vessel
Classification based on:
Anatomical location – ascending / descending
Duration of onset – acute / chronic
60-70% of aortic dissection involve ascending aorta & are acute in onset
Chronic dissection → almost always involve descending aorta
Most common disposing factors:
Hypertension
Marfan’s syndrome
Aortic Dissection - Classification
Type A
Originate in ascending aorta, usually within a few cm of aortic valve, and either
Extend into descending aorta (TYPE I), or
Limited to ascending aorta (TYPE II)
Type B (or TYPE III)
Involve only descending aorta; begins farther down aorta (beyond the arch), and extends into abdominal aorta.
Aortic Dissection - Etiology & Pathophysiology
Theory: attributes nontraumatic aortic dissection to degeneration of elastic fibers in medial layer
process accelerated by hypertension
intimal tear typically occur in the area with greatest rise in BP like immediately above the aortic valve and just distal to the left subclavian artery.
Affects 2-5X more in men
Predisposing factors: age, aortic diseases, atherosclerosis, blunt trauma, tobacco, cocaine or methamphetamine, Congenital heart disease (bicuspid aortic valve), connective tissue disorders (e.g., Marfan’s syndrome), family history
Aortic Dissection - Clinical Manifestations
Acute ascending aortic dissection
Sudden, severe, excruciating chest pain, back pain, or both, radiating to neck or shoulders – “sharp”, “worst ever”
Acute descending aortic dissection
pain back, abdomen, or legs
Aortic arch
May show neurological deficit e.g., altered LOC, weakened or absence of carotid and temporal pulses, dizziness, syncope
Ascending aortic dissection
usually causes some degree of disruption in coronary artery blood flow & aortic valve insufficiency → may cause angina, MI, etc.
Aortic Dissection - Management
Aortic dissection is medical emergency!
Once diagnosis of aortic dissection is suspected, treatment should begin immediately.
Type A dissections
High mortality - acute, more likely to rupture!!!
Requires surgery ⇒ involves replacement with a synthetic graft
Type B dissections
Best managed medically - chronic, non-life-threatening, can be managed w/ meds
1st line of treatment ⇒ management of hypertension with IV β-blockers
Goal is to rapidly ↓SBP, pulse pressure, and HR to minimize stress of dissection
Surgery is considered only if complications exist (i.e. rupture, renal or limb ischemia, uncontrollable hypertension, etc.
Aortic Dissection - Post-Op Nursing Care
Like that of aortic aneurysm repair so review post op care for AAA:
Pt in semi fowlers position and maintain quiet env.
HR and BP control along with pain management to reduce stress on the repaired dissection.
Morphine preferred as it decreases sympathetic stimulation
Coordinated Electrical Stimulation
Heart capable of automaticity
Two types of myocardial tissue
Contractile
Conductive
Impulses travel through ‘action potential superhighway’.
Electrical conduction in the heart proceeds mechanical contraction
if electricity isn't working the mechanical pumping is not effective = complication with cardiac output
Sinoatrial (SA) Node
Known as the primary pacemaker of the heart.
Composed of specialized cells that initiate electrical impulses.
Possesses an intrinsic rate that determines the heart’s rhythm.
Normal intrinsic rate: 60–100 bpm in most healthy adults.
If a myocardial infarction (MI) damages the SA node, the atrioventricular (AV) node assumes pacemaker control.
Atrioventricular (AV) Node
Functions as the secondary pacemaker.
Has its own intrinsic rate, which is slower than that of the SA node.
Intrinsic rate: 40–60 bpm
When the AV node takes over as pacemaker, P waves may appear inverted on the ECG.
Patients may become symptomatic when HR decreases from a normal 60 bpm to 40 bpm.
Symptoms of decreased CO: nausea, hypotension, and chest pain.
Ventricular Pacemaker (Backup)
If both SA and AV nodes fail, the ventricles generate impulses at a rate of 20–40 bpm.
Associated ECG findings: wide QRS complexes (compared to the narrow QRS seen with normal conduction).
Patients are typically very symptomatic but may still maintain some cardiac function.
Bundle block
Refers to an interruption or delay in the normal electrical conduction pathway within the heart.
Loss of conduction may occur due to:
Blocked blood vessel (e.g., clot or myocardial infarction): Prevents adequate blood flow and oxygen delivery to the cardiac tissue, impairing electrical conduction.
Electrocardiograms (ECGs)
Graphic recordings of the wave of electrical conduction across the myocardium.
Can help identify the location of a thrombus or area of ischemia within the heart.
P wave, QRS complex, T wave
P wave
Represents atrial depolarization (electrical activation of the atria).
Occurs when the SA node fires and the electrical impulse spreads through the atria, leading to atrial contraction.
QRS complex
Represents ventricular depolarization (electrical activation of the ventricles).
Occurs when the impulse travels through the bundle of His, bundle branches, and Purkinje fibers, leading to ventricular contraction.
A narrow QRS indicates normal conduction; a wide QRS suggests delayed or abnormal conduction (e.g., bundle branch block or ventricular rhythm).
T wave
Represents ventricular repolarization (recovery phase of the ventricles before the next contraction).
Abnormalities in the T wave (e.g., inversion, flattening, or peaking) can indicate ischemia, electrolyte imbalances, or other cardiac issues.
When the line doesn’t return to the isometric line = sign of MI - T-wave inversion
Dysrhythmias - Etiology
Some are asymptomatic.
Normal sinus rhythm on ECG → some pts dont require tx for dysrhythmias
Others require immediate treatment.
Occur in all age groups, in healthy and diseased hearts
Dysrhythmias - Symptoms
Dizziness
Angina
Weakness
Fatigue
Decreased exercise tolerance
Palpitations
Dyspnea
Syncope
Chest pain
Dysrhythmias - Associated Conditions/Diseases
HTN
Hyper/hypokalemia
MI
Stroke
Diabetes
Cardiac valve disease
HF
CAD
Dysrhythmias - Non-Pharmacological Treatment
Asymptomatic dysrhythmias
Little or no benefit to treatment with medications
Acute dysrhythmias
In life-threatening cases, medications warranted
Prophylaxis of dysrhythmias
Initiated for high-risk patients
Avoid drug combinations that increase QT interval
Antipsychotics / some abx
Cardioversion or defibrillation
Electrical stimulation of the heart reserved for serious types
Ablation = automaticity in cardiac cells, ectopic pacemaker firing above node
when cardioversion isn’t effective
tx for irregular arrhythmias by using heat or cold energy to create small scars in the heart to block the faulty signals = goal is to restore reg. HR
Identification and destruction of myocardial cells responsible for abnormal conduction
Cardiac pacemakers
Implantable Cardioverter Defibrillator (ICDs)
BRADY-dysrhythmias
HR less than 60 bpm
Common in older adults
Major indication for pacemakers
Common bradydysrhythmias:
Sinoatrial node dysfunction (sick sinus syndrome)
Atrioventricular (AV) conduction block
Pacemakers
They pace the atrium and/ or one or both ventricles
Internal: done under general anesthesia → wires go in and end up sitting in RA and in RV
Most pacemakers are “demand” pacemakers = activated when HR drops below a certain point, or heart skips a beat
Pacemakers can be temporary or permanent, and one sometimes precedes the other but not always.*
temporary (usually external) pacemaker used until drug effects wear off (beta-blockers, calcium channel blockers)
Pacemakers are “implanted” subcutaneously over the pectoral muscle on the pt’s non-dominant side.
Some patients with pacemakers are anticoagulated, but only <30% are.
Short-term, external cardiac pacing can be done in emergent situations until a pacemaker can be inserted or the underlying problem solved. This is known as transcutaneous pacing (or TCP) using defibrillator pads connected to a defibrillator, but at a much lower energy level.
Pre-Op Care of the Patient Receiving a Pacemaker
Medical hx - what led to this? risk factors? ECG/echo - any contraindications?
Evaluation of current condition (type of device to be implanted, the manufacturer, serial number, current settings required for proper functioning) - “pacemaker interrogation”
Evaluation of the ECG and Echocardiogram
If having a current pacemaker reimplanted, patients have an ID card providing info on brand, model, manufacturer, etc. This assists in selection of a new device.
Post-Op Care of the Patient Receiving a Pacemaker
Monitoring for complications (lead displacement, infection, pneumothorax)
Limiting arm movement on affected side x two weeks
Providing wound care
Ensuring discharge teaching (ie ID card/ medic alert bracelet, activity restrictions, signs of malfunction/ when to return for care, follow-up w/ cardiology, taking HR)
Follow-up Q 12 months w cardiology (or as directed)
NOTE: Patients with older dependent pacemakers should avoid MRI if possible. New models are safe, but this MUST BE CHECKED prior to patients going for MRI.
Implantable Cardioverter-Defibrillator (ICD)
A small device that constantly monitors heart rhythm and delivers electrical shocks to prevent cardiac arrest and dysrhythmias
Who benefits from an ICD? Patients who:
Have survived a sudden cardiac death (SCD)
Have spontaneous sustained ventricular tachycardia (VT)
Demonstrate syncope with inducible VT or fibrillation during electrophysiology study
Are at high risk for future life-threatening dysrhythmias
Use of ICDs have significantly decreased cardiac mortality rates among such patients
Follow-up after ICD insertion is usually Q6 months
TACHY-dysrhythmias
HR over 100 bpm
Incidence increases in older adults and those with preexisting cardiac disease.
Common tachydysrhythmias
Atrial tachycardia
Paroxysmal supraventricular tachycardia (PSVT)
Atrial flutter and fibrillation
Commonly treated w/ pharmacotherapy and / or synchronized cardioversion
Synchronized Cardioversion
What is it?
tx of choice for pts w hemodynamically unstable ventricular/superventricular tachydysrhymythias
done when hemodynamically unstable (decrease BP)
New presentations of Afib, symptomatic, new symptoms (worried about clots forming in atria)
How is it done?
defib. pads go on the pt->set the pacer pads to pick up on the R wave->we want the electrical shock to hit at the right point of the R wave
way less electricity than defibrillation
if worked = SA node working
heart will return to it’s normal pathway if we eliminate the ectopic source
Is it always effective? No
What if it doesn’t work? Go to CCU
NOTE: WHENEVER POSSIBLE (in the conscious patient), GIVE SEDATION PRIOR TO SHOCK, but don’t delay treatment in the hemodynamically unstable patient.
Unstable Angina (UA)
Chest pain that is:
new in onset
occurs at rest, or
has a worsening pattern
unpredictable
Chest pain that isn’t sustained
Constitutes a medical emergency
Chest pain results from myocardial ischemia
WOMEN EXPERIENCE UNIQUE SYMPTOMS: fatigue, SOB, indigestion and anxiety
ACS: Acute Coronary Syndrome
When myocardial ischemia is prolonged and not immediately reversible (due to narrowing or occlusion of the coronary arteries)
ACS is an umbrella term, that covers unstable angina, NSTEMI, and STEMI
***TIME IS MUSCLE*** - cardiac cells do not regenerate!
ACS - Nursing Assessment
Subjective Data
Health history
Symptoms – ask questions!
Objective Data
General – anxiety, fear, restlessness
Integumentary – cool, clamy, diaphoretic, pale/grey
Cardiovascular – tachy/bradycardia, dysrhythmias, BP changes
ACS - Goals of Care
For all ACS and stable angina patients the goal is the same:
1. Decrease the DEMAND for oxygen
2. Increase oxygen SUPPLY/ blood flow to the cardiac arteries
PRESERVE MYOCARDIUM!!!
ACS - Diagnostic Studies
12-lead ECG’s- STAT
Laboratory studies :
Urgently: Trops x 2 (3h apart) NOTE!
On admission: CBC, CP7, Fasting lipids and glucose, LFTs, BNP, TSH
Chest x-ray (non urgent)
Echocardiogram
Exercise stress test = done if everything is negative
NSTEMI: non ST elevated MI
---PARTIAL THICKNESS BLOCKAGE MI----
Majority of MI’s occur secondary to a thrombus formation
MI’s take time to damage the heart muscle
Takes 20 mins before cellular death starts to occur
Takes 5-6 hours before the entire thickness of the heart muscle becomes necrosed
Dead muscle DOES NOT rejuvenate
STEMI: ST Elevation MI
Total occlusion of a cardiac artery
-----FULL THICKNESS BLOCKAGE MI-----
What it looks like:
Can have the same symptoms as a NSTEMI, though usually more rapid onset, progression, and severity of symptoms.
Symptoms depend on the location of the blockage
Patients usually look “shocky” - shortness of breath, sweating, nausea, lightheadedness
Patients can have this feeling of “impending doom”
Generally look very unwell
GOALS: ECG within 10 minutes; ANGIOGRAM IN 90 MINS (“Door to balloon time: 90 minutes); if not possible = pt given thrombolytics (TNK), get sent to get better diagnosed
Complications of MI’s
Dysrhythmias: Present in 80% of patients who have had an MI
Heart Failure: The pumping action of the heart is diminished
Cardiogenic Shock: Inadequate O2 and nutrients are supplied to the tissues b/c of severe LV failure. Less common now d/t PCI.
Papillary Muscle Dysfunction: Causes mitral valve regurgitation (the valve between the LA and LV. This can reduce cardiac output (CO)
MI: Reperfusion Therapy
Emergent Percutaneous Coronary Intervention (PCI)
Angiogram
Part of cardiac catheterization
A procedure that uses contrast dye and fluoroscopy to examine blockages in coronary arteries
Angioplasty w/ stent
aka percutaneous coronary intervention (PCI)
Invasive treatment
Stenosis (narrowing) of coronary arteries are dilated with a balloon catheter
GOAL: Door-to-balloon= 90 mins
Fibrinolytic Therapy (ie TNK)
GOAL: Door-to-needle = 30 mins
Coronary Artery Bypass Graft (CABG)
Coronary Artery Bypass Graft (CABG)
Performed when one or more coronary arteries are blocked or narrowed due to plaque buildup (atherosclerosis), reducing oxygen delivery to the myocardium.
The goal is to bypass the blocked section of the coronary artery to improve blood flow and reduce symptoms such as angina, as well as prevent myocardial infarction.
A healthy blood vessel (usually taken from the saphenous vein in the leg, internal mammary artery, or radial artery) is grafted to create a new route for blood to flow around the blocked artery.
Benefits of PCI vs CABG
PCI (Percutaneous Coronary Intervention)
Alternative to surgery (a non-surgical procedure)
First choice
Performed with local anaesthetic
Pt is ambulatory within 24h
ELOS is 1-3 days post-PCI
Return-to-work 5-7 days post-PCI
CABG
Surgery
Performed with general anaesthesia
Pt can be ambulatory within 24h, but it’s usually longer
ELOS is 4-6 days post-CABG
Return-to-work is 2-8 wks post-CABG
CABG Post-op Nursing Care
Early mobilization (up at the side of the bed within 8h)
Wound care (sternum and from graft sites- arms or legs)
Detailed assessment of chest pain (ischemic vs related to thoracotomy) and aggressive management of same
chest will be sore = evaluate if it’s chest pain or chest soreness from surgery
Detailed assessment of dyspnea (pleural effusion vs hypoventilation r/t pain) and proactive management of same
These patients are at HIGH risk for atelectasis/pneumonia and VTE, therefore prevention is key as well as assessing for signs and symptoms of early complications.
NOTE: CABG requires heart-lung bypass intraop. This can lead to SIRS triggers. Be attentive to this and the alterations in vital signs so you can advocate.
Liver Structure
The liver is 1.2-1.6 kg in most adults and is the largest internal organ in the body.
It lies in the RUQ and is divided into R and L lobes.
Glisson’s capsule, which contains blood vessels, lymphatic vessels, and nerves, covers the liver.
The functional units of the liver are the lobules.
The lobule consists of plate of specialized hepatic cells called hepatocytes arranged around a central vein.
The capillaries or sinusoids are located between the plates of hepatocytes and are lined with Kupffer cells, which carry out phagocytic activity, removing 1. bacteria, 2. viruses, 3. parasites, 4. fungi, and other debris from the blood.
Kupffer cells also ingest aged red blood cells, breaking down Hgb into heme and globin. The heme is further broken down into iron and bilirubin, which is secreted into the bile.
Interlobar bile ducts form bile capillaries (canaliculi). The hepatic cells secrete bile into the canaliculi.
Portal Venous System
Liver receives both venous and arterial blood
⅓ of its blood flow comes from the hepatic artery (a branch of the celiac artery) = carries oxygenated blood
⅔ of liver’s blood supply comes from the portal vein - fed by blood flow form the spleen, intestines, stomach, and pancreas = partially oxygenated blood
Portal vein carries absorbed products from digestion from e.g. nutrients, metabolites, toxins (alcohol) directly to the liver where it comes in contact with each lobule and processes, detoxifies, and or assimilates those substances
Blood leaves the liver via hepatic vein and empties into the inferior vena cava
Functions of the Liver
Bile production
Bile helps the small intestine break down and absorb fats, cholesterol and some vitamins. Bile consists of bile salts, cholesterol, bilirubin, electrolytes and water2
Absorbs and metabolizes bilirubin
Bilirubin is formed by the breakdown of hemoglobin. The iron released from the hemoglobin is stored in the liver or bone marrow and used to make the next generation of blood cells
Assists in creating blood-clotting factors (coagulants)
Vitamin K is necessary to create certain coagulants, and to absorb vitamin K, bile is essential. Bile is created in the liver; if the liver does not produce enough bile, clotting factors cannot be produced
Fat metabolism
Bile breaks down fats to make them easier to digest
Carbohydrate metabolism
Carbs stored in the liver where they are broken down into glucose and siphoned into the bloodstream to maintain normal glucose levels. They are stored as glycogen and released whenever a quick burst of energy is required
Vitamin and mineral storage
Stores vitamins A, D, E, K and B12.
Iron from hemoglobin in the form of ferritin is stored in the liver, ready to make new red blood cells. The liver also stores copper and releases it when needed
Protein metabolism:
Bile helps break down proteins to make them digestible
Filters the blood
Liver filters and removes compounds from within the body, including hormones such as estrogen and aldosterone, and compounds from outside the body like alcohol and other drugs
Immunological function
Liver is part of the mononuclear phagocyte system. It contains high numbers of immunologically active cells called Kupffer cells; these cells destroy any pathogens that might enter the liver via the gut
Production of albumin
Most common protein in blood serum. It transports fatty acids and steroid hormones to help maintain the correct osmotic pressure and prevent blood vessels from becoming "leaky"
Synthesis of angiotensinogen
Raises blood pressure via vasoconstriction when alerted by the kidney's production of renin.
Cirrhosis
Liver cells attempt to regenerate.
Regenerative process is disorganized.
Abnormal blood vessel and bile duct formation
Overgrowth of new fibrous connective tissue distorts liver’s normal structure, impedes blood flow.
Irregular regeneration and disorganized regeneration, poor cellular nutrition and hypoxia d/t inadequate blood flow and scar tissue result in decreased liver functioning.
Cirrhosis is the final stage of chronic liver disease.
Factors that can lead to Cirrhosis
Chronic alcohol use disorder
Excessive alcohol ingestion is the single most common cause of cirrhosis.
Alcohol has a DIRECT hepatotoxic effect! It causes cell necrosis and fatty infiltration in the liver.
Some controversy continues as to whether the cause of cirrhosis is alcohol or the protein malnutrition that frequently coexists with chronic ingestion of alcohol
Nonalcohol fatty liver disease (NAFLD)
Cases of nutrition-related cirrhosis have resulted from extreme dieting, malabsorption, and obesity.
Patients with Hepatitis b and C
Environmental factors, as well as a genetic predisposition
Biliary cirrhosis
Associated with chronic biliary obstruction
Diffuse fibrosis of liver with jaundice
Cardiac cirrhosis
From longstanding severe right-sided heart failure
Cirrhosis - Clinical Manifestations
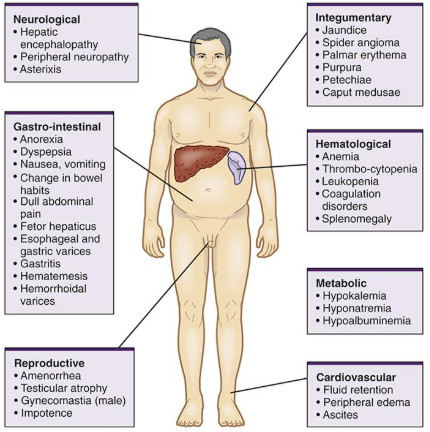
Common Assessment Findings for Hepatic Disorder
GI – clay colored stools, anorexia, N&V, constipation. This may be the first thing the patient comes to the doctor with.
Bilirubin levels rise – If it remains in the blood (can’t be broken down) it yields dark urine, yellow colored skin and sclera, icterus (yellow sclera), pruritis, fatigue, anorexia, allergies, and chemical sensitivities.
Energy deficits – fatigue and malaise. Carbohydrate, lipid and protein metabolism effected. Can also be anemia from bleeding due to impacted clotting factors.
Hemopoietic deficiency – lack of coagulation factors result in petechaie, bruising, and bleeding. Decreased Vit. K absorption.
Edema – pulmonary due to portal hypertension and dependent edema.
Ascites – lack of albumin causes intravascular leaks into the peritoneum.
Neurological changes – confusion, late signs due to build up of ammonia in the blood.
Physical changes – cachetic appearance, yellow skin, bruising, tired, dilated abdominal veins (portal hypertension), protruding abdomen (ascites); esophageal varicies – risk for uncontrolled hemorrhage.
Vitamin K deficiency is manifest as a tendency to bleed excessively. Indeed, many commercially-available rodent poisons are compounds that interfere with vitamin K and kill by inducing lethal hemorrhage.
Cirrhosis - Skin Lesions
Spider angioma occurs on the nose, cheeks, upper trunk, neck, and shoulders.
Palmar Erythema a red area that blanches with pressure appears on the palms of the hands.
Both of these are caused by low levels of circulating estrogen as a result of the liver’s inability to metabolize steroid hormones.
Cirrhosis - Peripheral Neuropathy
Found in alcoholic cirrhosis, probably caused by a deficiency in thiamin, folic acid, Vitamin B12
Peripheral neuropathy is damage to nerves outside the brain and spinal cord.
Causes weakness, numbness, and pain, usually in the hands and feet, but can affect other areas.
Can result from traumatic injuries, infections, metabolic problems, inherited conditions, or toxin exposure.
Diabetes is one of the most common causes.
Pain is often described as stabbing, burning, or tingling.
Cirrhosis - Endocrine Disorders
Gynecomastia is a non-cancerous enlargement of male breast gland tissue caused by increased estradiol levels.
Hypogonadism is a decrease in male sex hormones.
Symptoms include impotence, infertility, loss of sexual drive, and testicular atrophy.
It can result from primary gonadal injury or suppression of hypothalamic/pituitary function.
Hypogonadism is often associated with cirrhosis due to alcoholism or hemochromatosis.
Cirrhosis - Hematological Problems
Thrombocytopenia
Decreased thrombopoietin (TPO) - a hormone produced by the liver and kidney that regulates platelet production in the bone marrow.
Leukopenia
Anemia
Decreased Prothrombin. What problem would you expect to see with this?
a liver-made protein essential for blood clotting
Liver Function Tests
ELEVATED liver enzymes: ALT, AST, LDH, Alk Phos, GGT
ALT to the liver is what troponins are to the heart - shows damage to liver cells
AST found in different parts of body
GGT spike with binge drinking - acute alcohol episodes
DROP in serum albumin levels (normal 3.5 – 5.0 G/dl)
May see peripheral edema, ascites
INCREASE prothrombin time/ PT ( normal 11-15 sec.); INR trends similar to the PT
Prothrombin made in the liver
Unconjugated bilirubin (fat soluble, indirect) - too much bilirubin in the blood.
Conjugated bilirubin (water soluble, direct) - impaired excretion
Cirrhosis - Collaborative Care
Rest
Avoidance of alcohol, Aspirin, acetaminophen, and NSAIDs
Prevention and management of esophageal variceal bleeding
Management of ascites
Management of encephalopathy
Wernicke’s
Hepatic
Portal Hypertension
An increase in the blood pressure within the system of veins called the portal venous system
Veins coming from the stomach, intestine, spleen, and pancreas merge into the portal vein, which then branches into smaller vessels and travels through the liver.
If the vessels in the liver are blocked due to liver damage, blood cannot flow properly through the liver. As a result, high pressure in the portal system develops.
This increased pressure in the portal vein may lead to the development of large, swollen veins (varices) within the esophagus, stomach, rectum, or umbilical area (belly button).
Varices can rupture and bleed, resulting in potentially life-threatening complications
Esophageal Varices - Clinical Manifestations
Vomiting blood
Black, tarry or bloody stools
Shock (in severe case)
Esophageal Varices - Diagnosis
Endoscope exam - upper gastrointestinal endoscopy (preferred method)
Imaging tests
Both abdominal CT scans and Doppler ultrasounds of the splenic and portal veins can suggest the presence of esophageal varices.
Capsule endoscopy
Esophageal Varices - Treatment (goal, prevent bleeding, if bleeding, prevent rebleeding)
Primary goal: Prevent esophageal variceal bleeding, which is life-threatening.
Treatment to prevent bleeding:
Lower portal vein pressure: Medications such as beta blockers (propranolol, nadolol) reduce portal vein pressure.
Esophageal band ligation - varices are tied off with elastic bands to prevent bleeding
Treatment if bleeding occurs:
Esophageal band ligation to stop active bleeding.
Octreotide reduces blood flow to portal vein, usually continued for 5 days.
Divert blood flow (TIPS procedure):
Shunt between portal vein and hepatic vein reduces portal pressure.
Used when other treatments fail or as a bridge to liver transplant.
Complications: liver failure, mental confusion.
Restore blood volume: Blood transfusions and clotting factors may be given.
Abx to prevent infection
Liver transplant may be required for diseased liver replacement.
Preventing rebleeding:
Beta blockers and esophageal band ligation are recommended to reduce recurrence.
Esophageal Varices - Collaborative Care
Supportive measures for acute bleed
Fresh-frozen plasma
Packed RBCs
Vitamin K
Proton pump inhibitors
Octreotide
Stops bleeding by vasoconstricting the splanchnic circulation
This reduces added pressure on the portal venous system
Octaplex (in severe cases)
Ascites
Ascites is the abnormal accumulation of fluid in the peritoneal cavity, commonly seen in advanced liver disease/cirrhosis.
Ascites is primarily a consequence of portal hypertension, reduced albumin synthesis, and sodium and water retention in the kidneys.
The portal hypertension rises pressure in the liver’s sinusoids forcing fluid to leak from the blood into the abdominal cavity.
The increased pressure also triggers the release of chemical factors like nitric oxide which dilates blood vessels and lowers blood volume elsewhere which can activate sodium and water retention in the kidneys leading to additional fluid accumulation.
Portal hypertension + low albumin + renal sodium/water retention → ascites.
Ascites - Clinical Manifestations
Abdominal Distention +/- umbilical eversion
Weight gain
Abdominal striae
Decreased urine output
Signs of dehydration
Hypokalemia
Ascites - Treatment
Focused on sodium restriction, diuretics, and fluid removal
Diuretics
First choice: Spironolactone - aldosterone agonist and low-dose Furosemide
Spironolactone blocks the effects of the hormone aldosterone, causing the kidneys to excrete more salt and water through urine. It is a first line treatment for ascites along with a 2g/ day Na+ restriction.
Paracentesis
Removes fluid from abdominal cavity
Temporary measure
Continuous reinfusion of ascitic fluid from the abdomen to the vena cava
Not first-line therapy
Relief provided is only temporary.
Hepatic Encephalopathy
Most ammonia in the body forms when protein is broken down by bacteria in the intestines.
The liver normally converts ammonia into urea, which is then eliminated in urine.
Ammonia levels in the blood rise when the liver is not able to convert ammonia to urea.
This may be caused by cirrhosis or severe hepatitis.
Hepatic Encephalopathy - Clinical Manifestations
Altered LOC
Neuromuscular changes
***Asterixis - tremor of the hand when the wrist is extended
Hyperreflexia exaggeration of reflexes
Hepatic Encephalopathy - Treatment
Goal: Reduction of ammonia formation
Lactulose
Can be given PO or PR
It is a colonic acidifier that works by decreasing the amount of ammonia in the blood.
Usual dosing varies but is titrated per LOC and to produce 2-3 soft BMs/ 24h. Often this requires Lactulose 15-30mL TID-QID.
Patient/ caregiver teaching:
Daily adherence to Lactulose regimen
Monitoring # of BM’s/ day
Monitoring for GI bleeds (which increases risk for hepatic encephalopathy and might increase dose of Lactulose)
Antibiotics
Treatment of precipitating cause (ie controlling GI hemorrhage to decrease protein in the GI tract)
Liver Transplant
Drugs and Liver Function
Avoid hepatotoxic drugs like Acetaminophen, ASA
First Pass effect
This affects ORAL meds and they should be given in HIGHER doses.
Use caution in administering medications in patients with advanced liver disease.
CIWA-Ar (Purpose & Scoring)
Purpose
Used to assess the severity of alcohol withdrawal in patients with a history of alcohol abuse.
Guides medication management and monitoring during withdrawal.
How to Use CIWA-Ar:
Scoring
0–8: Minimal or absent withdrawal
9–15: Mild withdrawal
16–20: Moderate withdrawal
>20: Severe withdrawal, may need close monitoring or ICU care
Key Factors Assessed on CIWA
Nausea and vomiting
Tremor (hands)
Paroxysmal sweats
Anxiety
Agitation
Tactile disturbances (itching, pins and needles, burning)
Auditory disturbances (hallucinations, sounds)
Visual disturbances (hallucinations, lights)
Headache or fullness in head
Orientation and clouding of sensorium
Timing of Alcohol Withdrawal
Symptoms onset: Usually 6–24 hours after the last drink.
Peak symptoms: Typically 24–72 hours after cessation.
Severe complications (delirium tremens, seizures) usually occur 48–72 hours after last drink.
Hepatitis A
Sources of infection | Infectivity | Treatment |
- Fecal-oral route (contaminated food/water) - Close personal contact with an infected person | Moderate; highly infectious during 2 weeks before symptom onset | - Usually self-limiting; supportive care - Hydration, rest, nutrition - Prevention: HAV vaccine, hand hygiene, safe food practices |
Hepatitis B
Sources of infection | Infectivity | Treatment |
- Blood, semen, body fluids - Perinatal (mother to child at birth) - Sexual contact - Needle sharing / contaminated medical equipment | Highly infectious; chronic infection possible | - Acute HBV: supportive care - Chronic HBV: antiviral therapy (e.g., tenofovir, entecavir) - Prevention: HBV vaccine, safe sex, avoiding needle sharing |
Hepatitis C
Sources of infection | Infectivity | Treatment |
- Blood exposure (IV drug use, transfusions before 1992, contaminated needles) - Rarely sexual or perinatal | Moderate; chronic infection common | - Direct-acting antivirals (DAAs) → cure rates >95% - No vaccine currently available - Avoid alcohol and hepatotoxic drugs |
Liver Cancer
Hepatocellular Carcinoma (HCC)
Third most common cancer in the world
Causes:
80-90% of HHC patients have cirrhosis
Chronic HCV or HBV
NAFLD
Clinical manifestations:
Similar to cirrhosis; minimal in early stages
Treatment: surgical removal or palliation
Focus of care: symptom management
Liver Resection
Typical surgical procedure to remove up to 2/3 of a patient’s liver, usually due to HCC
Remaining liver tissue can regenerate and grow back to its normal size within a few months if the remaining tissue is healthy.
Open vs laparascopic approaches
General anaesthesia
Complications:
Infection, jaundice and bleeding (immediate post-op phase)
Altered liver function if bleeding persists.