Vesper geometrices
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
34 Terms
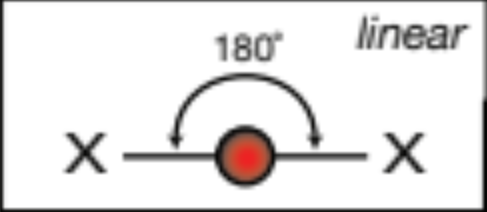
AX2; 2 atoms, 0 lone pairs
linear, 180 degrees
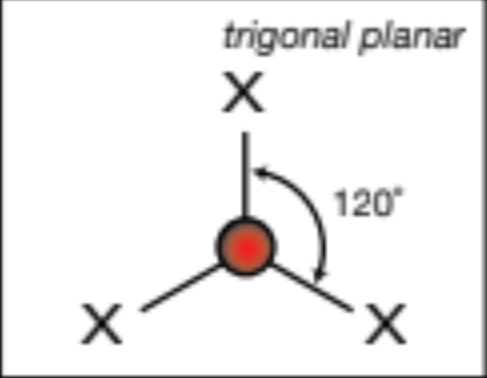
AX3; 3 atoms 0 lone pairs
trigonal planar, 120 degrees
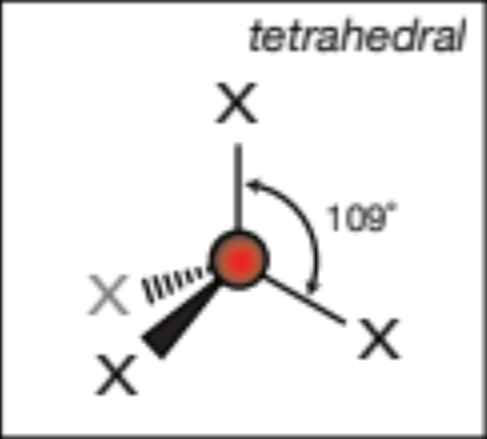
AX4; 4 atoms, 0 lone pairs
tetrahedral, 109.5 degrees
AX5; 5 atoms, 0 lone pairs
trigonal bipyramid, 90 degrees between vertical axis, 120 degrees horizontal axis
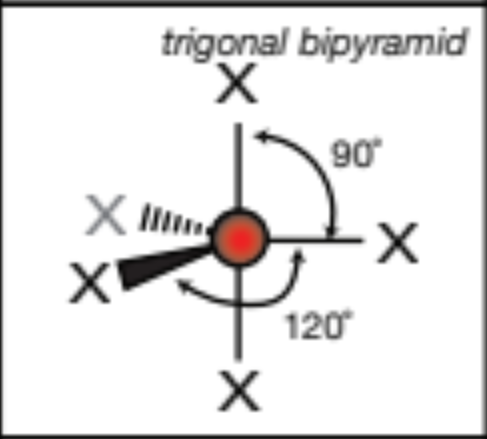
AX6; 6 atoms, 0 lone pairs
octahedral, 90 degrees
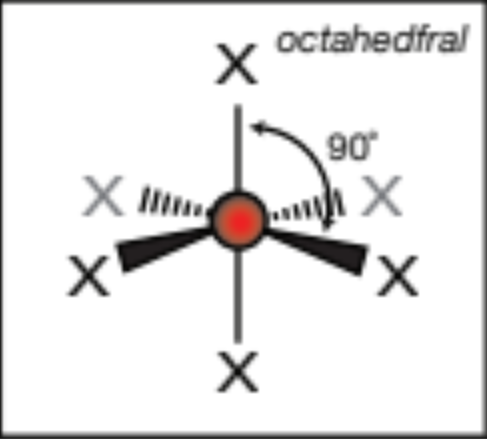
AX2E, 2 atoms, 1 lone pair
bent, 120 degrees
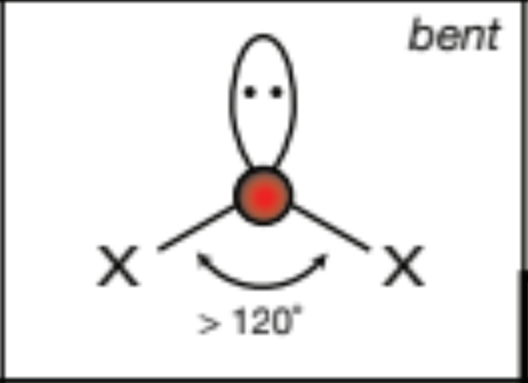
AX3E, 3 atoms, 1 lone pair
trigonal pyramid, 109.5 degrees
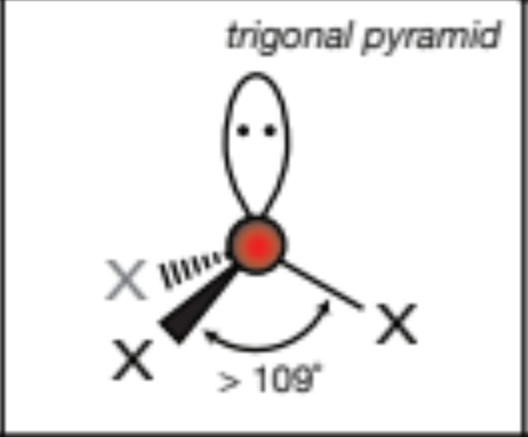
AX4E, 4 atoms, 1 lone pair
seesaw, 90 degrees on vertical axis, 120 degrees on horizontal axis
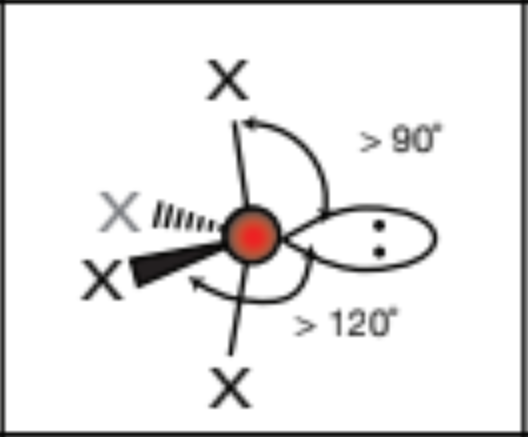
AX5E, 5 atoms, 1 lone pair
square pyramid, 90 degrees
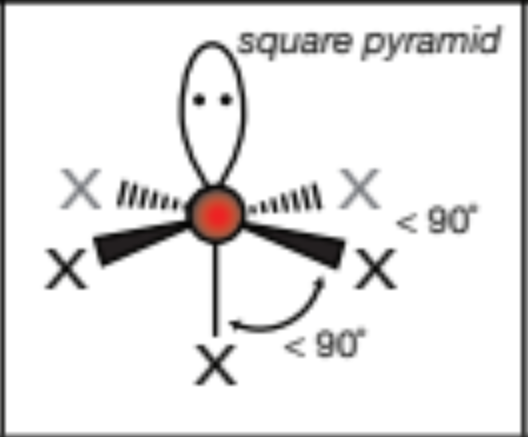
AX2E2, 2 atoms, 2 lone pairs
bent, 120 degrees
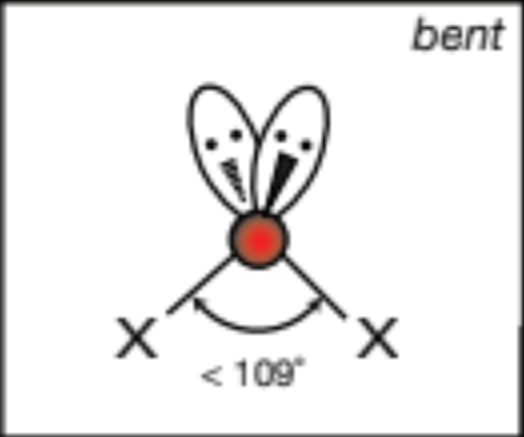
AX3E2, 3 atoms, 2 lone pairs
T-shaped, 90 degrees
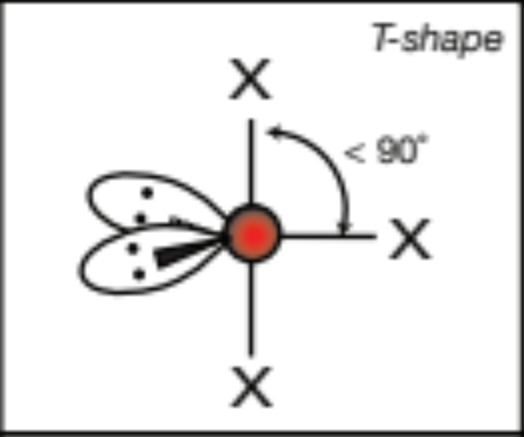
AX4E2, 4 atoms, 2 lone pairs
square planar, 90 degrees
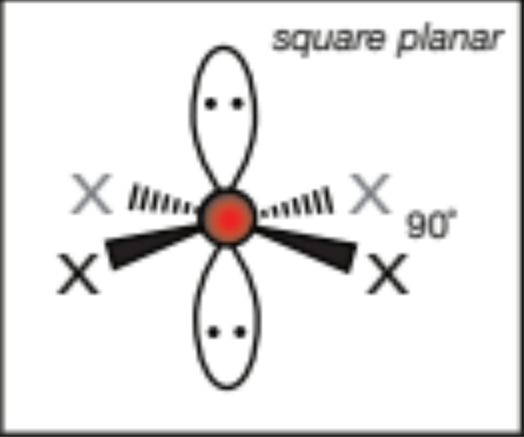
AX2E3, 2 atoms, 3 lone pairs
linear, 180 degrees
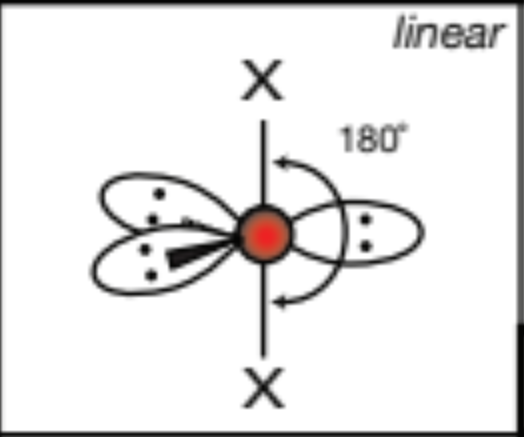
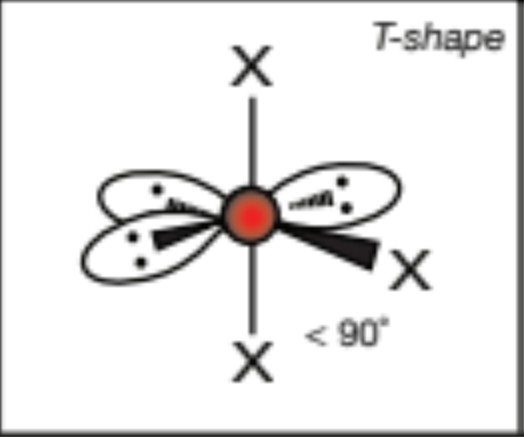
AX3E3, 3 atoms, 3 lone pairs
T-shaped, 90 degrees
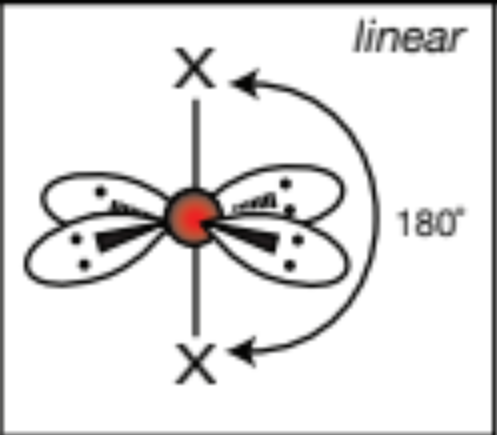
AX2E4, 2 atoms, 4 lone pairs
linear, 180 degrees
Why are the degrees of bonds in certain VSEPR models smaller than expected?
Lone pairs push bonds away the bonds. Repulsion. :)
Which 2 atoms don’t need a full octet? (besides H and He)
Boron and Beryllium
What makes a stronger bond?
Better orbital overlap
2 electron-groups: electron group geometry?
linear
3 electron-groups: electron group geometry?
trigonal planar
4 electron-groups: electron group geometry?
tetrahedral
5 electron-groups: electron group geometry?
trigonal bipyramidal
6 electron-groups: electron group geometry?
octahedral
Electron group geometry
Describes how all electron-group are arranged around a central atom. Does not differentiate between lone pairs and bonds.
Molecular geometry
Describes how bonded atoms are oriented around a central atom.
When are pi bonds delocalized?
When the bond has resonance across multiple atoms.
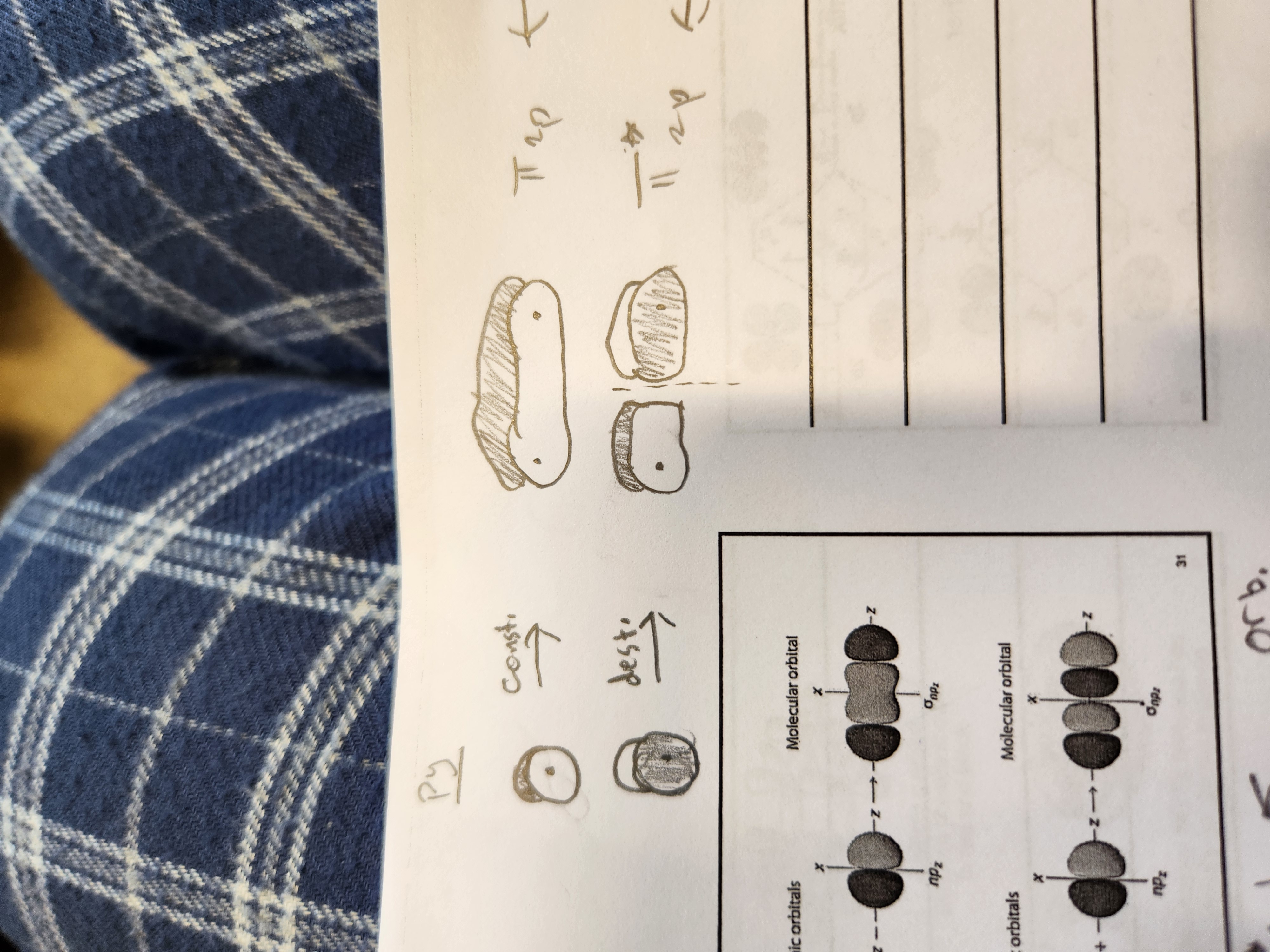
What type of bonds result from combining orbitals of the same phase or end-to-end?
constructive
What type of bonds result from combing orbitals of a different phase or not end-to-end
destructive
Bonding Energy equation
(# bonding e- - # antibonding e-)/2
Pz MO orbitals
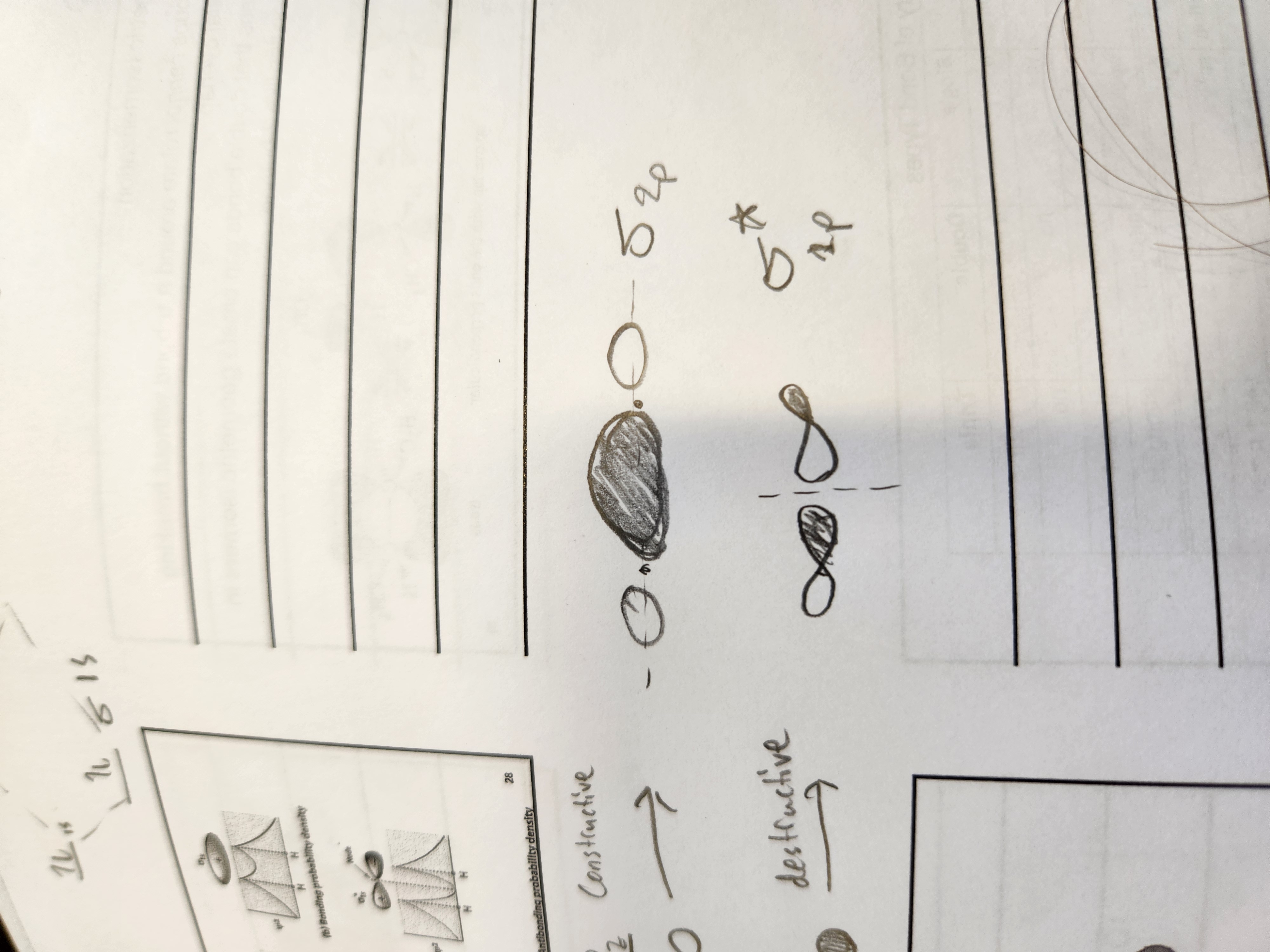
Px MO
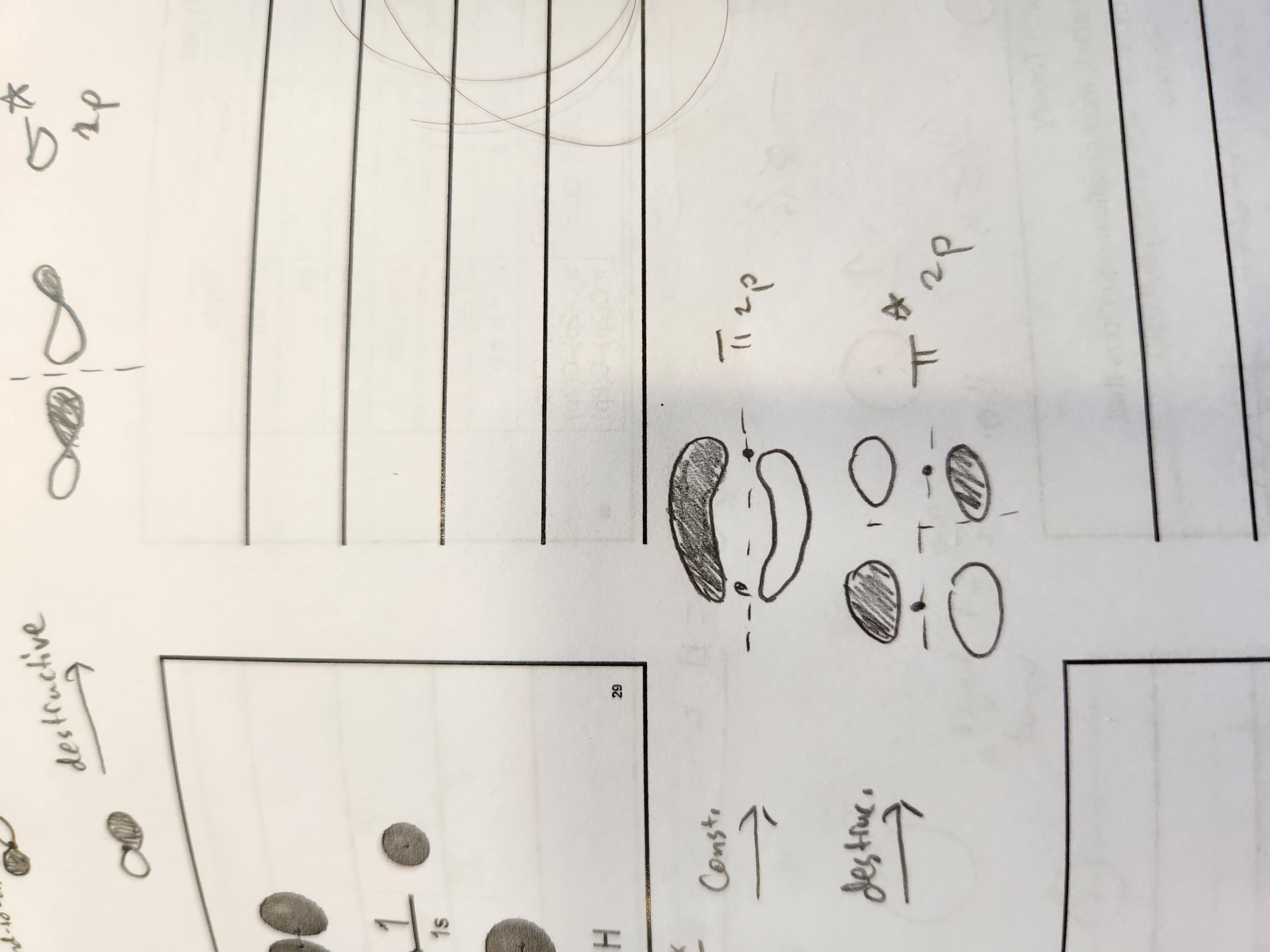
Py MO