Chemistry Midterm
5.0(1)
Card Sorting
1/53
Last updated 4:20 PM on 1/18/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
1
New cards
Qualitative observations
Based on your senses and observations
2
New cards
quantitative observation
Involves numbers and measurements
3
New cards
All parts of a lab write-up (in order)
Title, purpose, procedure, data and observations, calculations, conclusion and questions
4
New cards
Intensive properties
do not depend on the amount of matter present
5
New cards
Extensive properties
depend on the amount of matter that is present
6
New cards
Physical vs. chemical change
Physical - does not create a new substance, atoms are not rearranged into different compounds; Chemical - converts one substance into another; atoms are rearranged
7
New cards
compounds
2 or more different elements chemically combined; composition always remains the same
8
New cards
elements
A molecule composed of one kind of atom; cannot be broken into simpler units by chemical reactions.
9
New cards
homogeneous material
an item that consists of only one material throughout or a combination of multiple materials that cannot be mechanically disjointed, excluding surface coatings
10
New cards
heterogeneous material
a material with a composition that varies from one point to the next within its structure
11
New cards
mixtures
A combination of two or more substances that are not chemically combined and have varying composition (wood, soda, coffee, air)
12
New cards
molecules
Groups of two or more atoms held together by chemical bonds
13
New cards
Difference in melting points for pure substances vs mixtures
Pure substances have definite melting points; mixtures do not
14
New cards
Methods of separating mixtures
filtration, distillation, crystallization, chromatography, magnet, sorting by color, centrifuging
15
New cards
Characteristic of solids and the atoms/molecules within them
Have definite shape and volume, atoms are tightly packed and vibrate in place, strong force of attraction between particles
16
New cards
Characteristic of liquids and the atoms/molecules within them
definite volume but no definite shape, flow, looser attractive forces
17
New cards
Characteristic of gases and the atoms/molecules within them
no definite volume or shape, weak attractive forces, flow
18
New cards
Physical property
Characteristic that can change without turning it into a different substance (odor, volume, state, density, melting point, boiling point)
19
New cards
Chemical property
Characteristic that describes the ability of a substance to change to a different substance
20
New cards
Physical change
change that does not affect a substance's composition
21
New cards
chemical change
substance becomes another substance (wood burning, rusting of steel, digestion of food, growth of plants)
22
New cards
Alloy
mixture of a metal
23
New cards
pure substances
always have the same composition (elements or compounds)
24
New cards
Matter
Must have mass and volume
25
New cards
Mass vs. weight
Mass is a measurement of the amount of matter something contains (makeup stays the same everywhere), while weight is the measurement of the pull of gravity on an object (changes on different planets).
26
New cards
Colloids
heterogeneous, shows Tyndall effect, will not settle out over time, think snickers bar or tapioca pudding
27
New cards
Suspensions
heterogeneous, shows Tyndall effect, settles out over time
28
New cards
Solutions
Homogeneous, no definite formula, type of mixture
29
New cards
Scientific method
1. Observations 2. Formulate hypothesis 3. Experiment -- Theory, prediction, experiment repeat enough and could end up with a law
30
New cards
Relationship between temperature and pressure
As temperature increases, so does pressure, and as it decreases, so does pressure (because temperature increases particle movement, they move more often and therefore hit the sides of things more, increasing pressure)
31
New cards
Percent composition by mass
part/whole x 100
32
New cards
Empirical formula
Expressing the smallest whole number ratio of atoms in a compound
33
New cards
Molecular formula
The actual formula of a compound
34
New cards

What is this and what is it for?
This is a test tube, and its purpose is to hold, mix, or heat small quantities of substances
35
New cards

What is this and what is it for?
This is an iron ring, and its purpose is to hold up whatever is placed on the Bunsen burner
36
New cards

What is this and what is it for?
This is a ring stand and it holds an iron ring. Commonly, a Bunsen burner is placed beneath it to heat whatever rests on it
37
New cards

What is this and what is it for?
This is a test tube rack and its purpose is to hold test tubes
38
New cards
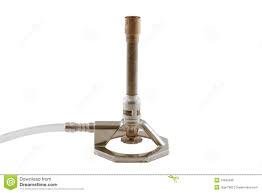
What is this and what is it for?
This is a Bunsen burner and its purpose is to heat things
39
New cards

What are these and what are they for?
These are crucible tongs and they hold the crucible
40
New cards

What are these and what are they for?
These are beaker tongs and they hold the beaker
41
New cards

What is this and what is it for?
This is a dropper pipette and its purpose is to transfer small amounts of liquid
42
New cards

What is this and what is it for?
This is a watch glass and is typically used for evaporation and weighing solids
43
New cards

What is this and what is it for?
This is a glass stirring rod and it is for stirring solutions/mixtures
44
New cards

What is this and what is it for?
This is a thermometer and it is for checking the temperature of something
45
New cards

What is this and what is it for?
This is a scoopula and it is for scooping small amounts of things and removing/scraping substances
46
New cards

What is this and what is it for?
This is a test tube holder and its purpose is to carry/hold test tubes
47
New cards

What is this and what is it for?
This is a pipestem triangle and its purpose is to hold up the crucible while it is being heated above the Bunsen burner
48
New cards

What is this and what is it for?
This is a crucible and cover, and its purpose is to hold, typically solids, to give them somewhere to be while they are being burned/heated over a Bunsen burner
49
New cards

What are these are what are they for?
These are forceps and they help hold/carry small things
50
New cards

What are these and what are they for?
This is a mortar and pestle and they are for grinding/crushing solids
51
New cards

What is this and what is it for?
This is a graduated cylinder and its purpose is to measure amounts of liquid in milliliters
52
New cards

What is this and what is it for?
This is an evaporating dish and it is used to allow things to heat/evaporate over time
53
New cards

What is this and what is it for?
This is a wire gauze and it is for protecting glassware on a Bunsen burner by diffusing the heat
54
New cards

What is this and what is it for?
This is a beaker and it is for measuring liquids, containing liquids/solids, and for holding things that may be used in a reaction