9.4, 9.5 Uses of Metals, Extraction of Metals
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Metal | Property | Use |
Aluminium, Al | Low d__ | A__ |
Resistant to c__ | F__ containers | |
Low density, good electrical conductor | Overhead electrical c__ |
density, aircraft, corrosion, food, cable
Metal | Property | Use |
Copper, Cu | Good electrical c__, ductile | Electrical w__ |
Good h__ conductivity | P__ & pans | |
conductor, wires, heat, pots
Metal | Property | Use |
Zinc, Zn | Higher than Fe in reactivity | G__ |
galvanising
Mild steel is made up of mostly F__, some C
USE: machinery
fe
Stainless steel is made up of mostly F__; Cr, Ni
USE: c__
fe, cutlery
Extraction
Metal | Method |
K |
E__ |
Zn | Reduction of sulfide by O2 |
Fe | B__ furnace (reduced by CO) |
Pb | Reduction by __ (think abt reactivity) |
Cu | Reduction by __ (think abt reactivity) |
Ag | Natural state |
electrolysis, blast, C, H
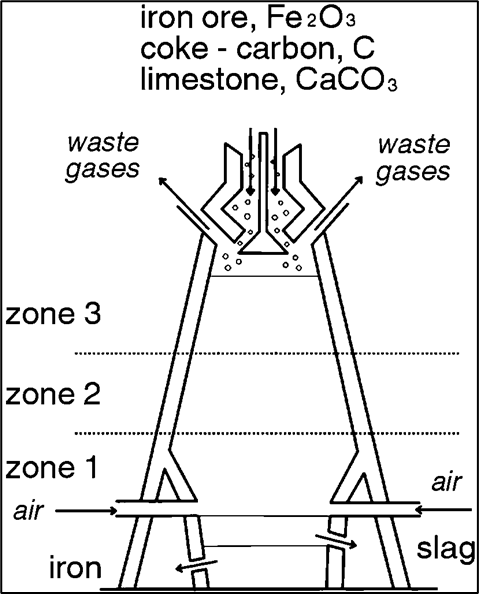
Which zone has the highest temp?
1
Extraction of Iron (Blast Furnace)
1. Feed iron ore (h__), c__, l__ into furnace
2. H__ air reacts w/ c__:
> C + O2 → __
hematite, coke, limestone, hot, coke, co2
Extraction of Iron (Blast Furnace)
3. Add more coke:
> CO2 + __ → 2CO
> The “2CO” is the r__ agent
C, reducing
Extraction of Iron (Blast Furnace)
4. Reduce (extract) iron from its ore:
Fe2O3 + 3__ → 2Fe + 3CO2
CO
Extraction of Iron (Blast Furnace)
5. Decompose l__ w/ heat:
> CaCO3 → C__ + CO2
CaO w/ impurites form s__:
> CaO + S__ → C__
6. iron tapped
limestone, CaO, slag, SiO2, CaSiO3
Is the iron output liquid or solid?
liquid
Limestone is added to:
> make C__
> remove S__ (impurities) from the furnace
co2, sio2