PHAR 501 Final Exam (Review)
1/85
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
86 Terms
_____ ft = _____ in
1 ft = 12 in
_____ in = _____ cm
1 in = 2.54 cm
_____ fl oz = _____ mL
1 fl oz = 29.6 mL
_____ cup = _____ oz
1 cup = 8 oz
_____ pint = _____ cups = _____ mL
1 pint = 2 cups = 473 mL
_____ quart = _____ pints = _____ mL
1 quart = 2 pints = 946 mL
_____ gallon = _____ quarts = _____ mL
1 gallon = 4 quarts = 3785 mL
_____ tsp = _____ fl oz = _____ mL
1 tsp = 1/6 fl oz = 5 mL
_____ tbsp = _____ fl oz = _____ mL
1 tbsp = ½ fl oz = 15 mL
_____ cc = _____ mL
1 cc = 1 mL
_____ lb = _____ oz = _____ g
1 lb = 16 oz = 454 g
_____ kg = _____ lb
1 kg = 2.2 lb
_____ grain = _____ mg
1 grain = 65 mg
pico (p)
10-12
nano (n)
10-9
micro (mc, μ)
10-6
milli (m)
10-3
centi (c)
10-2
deci (d)
10-1
deca (da)
101
hecto (h)
102
kilo (k)
103
mega (M)
106
giga (G)
109
tera (T)
1012
percentage → decimal
Percent/100 = Decimal
decimal → percentage
Decimal * 100 = Percentage
rules for rounding
Only 1 uncertain figure
Next digit < 5 → Round down
Next digit > 5 → Round up
Use logic
If we get a decimal for the average number of patients, we would typically want to round up to slightly overrepresent the data. It’s better than underrepresenting
0.1 ←
0.01 ←
0.001 ←
0.0001 ←
0.1 ← Tenths place
0.01 ← Hundredths place
0.001 ← Thousandths place
0.0001 ← Ten thousandths place
density
Density of water?
Density of water = 1 g/cc = 1 g/mL
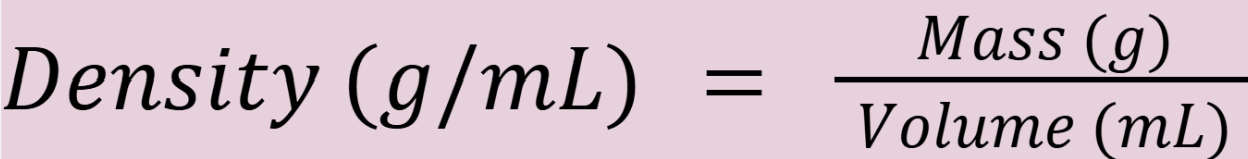
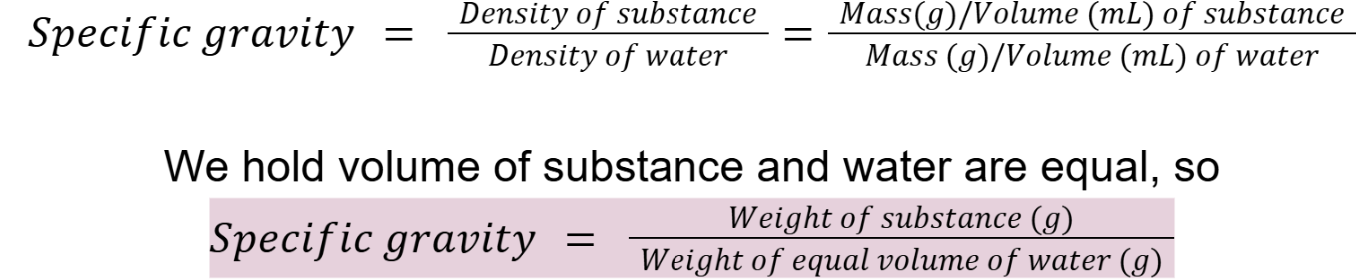
specific gravity
Shortcut?
Specific gravity of water?
Specific gravity of water = 1
specific gravity via pyncometer
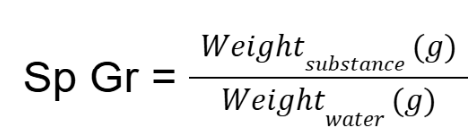
mass of substance using specific gravity
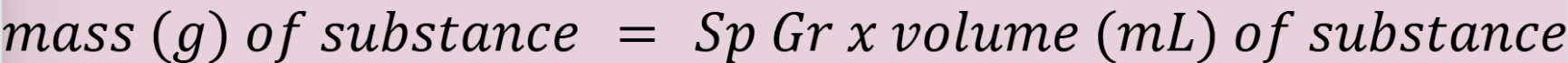
volume of substance using specific gravity

drug dosing by body weight

drug dosing by BSA
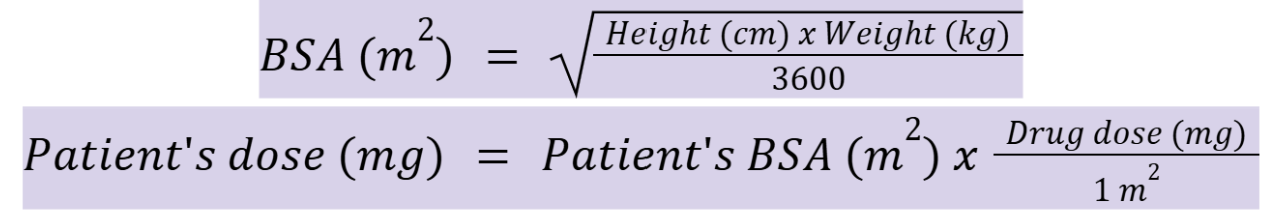
creatinine clearance for males (CrClmales)

creatinine clearance for females (CrClfemales)
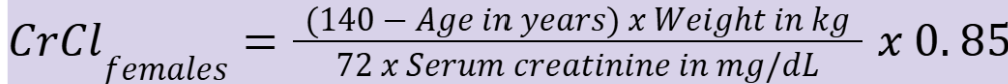
What is considered normal CrCl?
> 90 mL/min
ideal body weight for males (IBWmales)
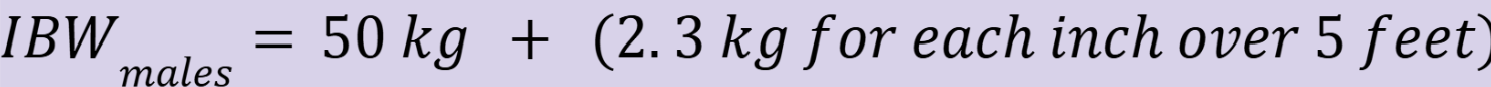
ideal body weight for females (IBWfemales)

adjusted body weight (AdjBW)

calculation rules for day supply
Divide the total amount being dispensed by the most medication a patient can take in one day
Day supply should always be expressed as a whole number, not a fraction
Round down for day supply
BUD trumps the day supply
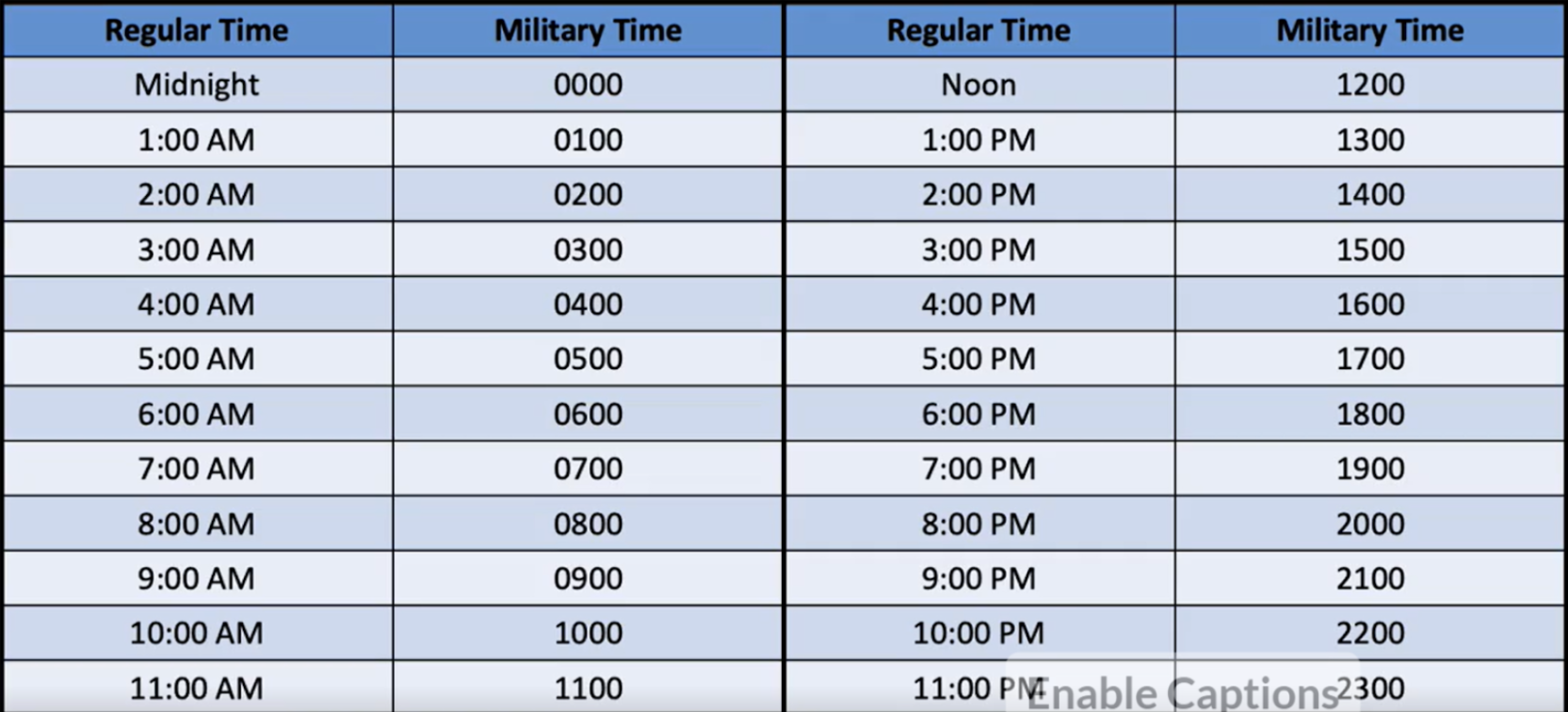
tips for converting to and from military time
To convert standard to military time, add 12 to the hour for any time from 1:00 PM to 11:59 PM
To convert military to standard time, subtract 12 from any hour after 1200
percent weight in volume (units)
Example: 5% w/v = 5 g/100 mL
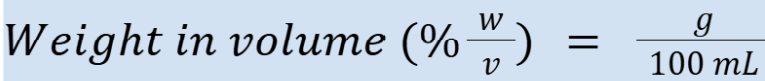
percent volume in volume (units)
Example: 10% = 10 mL/100 mL
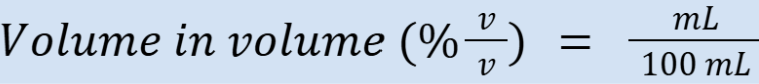
percent weight in weight (units)
Example: 20% = 20 g/100 g
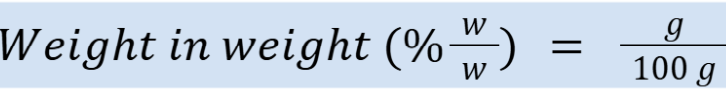
What is ratio strength always expressed as?
Solids in solids (w/v) = _____ → _____
Liquids in liquids (v/v) = _____ → _____
Solids in solids (w/w) = _____ → _____
Always expressed as 1:x (e.g., 10% = 10:100 → 1:10)
Solids in liquids (w/v) = 1:x → 1 g in x mL
Liquids in liquids (v/v) = 1:x → 1 mL in x mL
Solids in solids (w/w) = 1:x → 1 g in x g
parts per million (PPM)
1 part of the agent per 1 million
1 L = 1000 mL = 1000 g (via density of water) = 1,000,000 mg
parts per billion
1 part of the agent per 1 billion
What concentration in percent does a powder have?
100%
What concentration in percent does a diluent, like water, or dextrose injection have?
0%
process for calculating pharmaceutical strength
Set up your 3×3 alligation table
Highest concentration (A%) in top left, lowest concentration (B%) in bottom left, desired concentration (C%) in the middle
All the values need to be in % strength
Cross subtract (literally X)
C - B in top right
A - C in bottom right
Read your alligation table straight across to get the relationship for your two components
C - B parts of A% (# of grams, mL, etc.) in top right
A - C parts of B% (# of grams, mL, etc.) in bottom right
Find the total parts
May only be necessary if the concentration or quantity of the desired product is necessary for solving the problem
Total parts = (C - B) + (A - C) parts of C% (# of grams)
Use proportions to find your answers
Keep pairs of information together (in the same fraction!)
freezing point of blood serum and lacrimal fluid
-0.52 °C
What is the freezing point of 1 g of molecular weight of a compound that is not an electrolyte when dissolved in 1000 g of water (to make an isotonic solution)?
-1.86 °C
Calculating tonicity for electrolytes is more complicated because the osmotic pressure depends on the…?
number of particles that dissociate
Dissociation factor is symbolized by i. What is the i for the following substances:
Non-electrolytes and substances of slight dissociation: _____
Substances that dissociate into 2 ions: _____
Substances that dissociate into 3 ions: _____
Substances that dissociate into 4 ions: _____
Substances that dissociate into 5 ions: _____
1
1.8
2.6
3.4
4.2
simple isotonic solutions
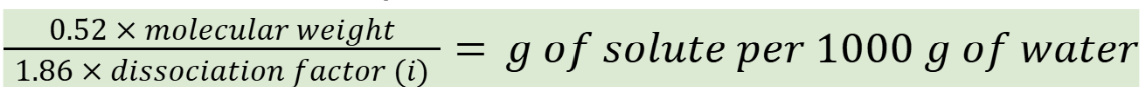
E-value of a substance
If you aren’t given the E-value of substance, you will be given its MW and i (if not obvious) to calculate E-value
MWNaCl = 58.5
iNaCl = 1.8
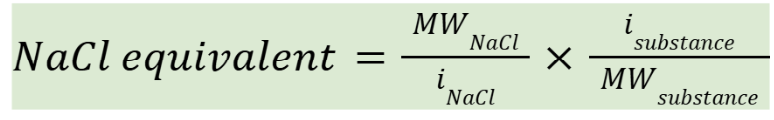
approach for E-value questions
Calculate the amount of NaCl represented by each ingredient in a prescription by multiplying the amount of each ingredient by its sodium chloride equivalent (E-value)
If given a percent strength, you’ll need to convert it to a fraction of units (e.g., 2% w/v = 2 g/100 mL)
Calculate the amount of NaCl, alone, that would be combined in an isotonic solution of the volume specified in the prescription (specifically, the amount of NaCl in a 0.9% solution of the specified volume)
Step 2 - Step 1 = amount of NaCl to be added to make the solution isotonic
If an agent other than NaCl (i.e., boric acid, dextrose, mannitol) is to be used to make a solution isotonic, divide Step 3 by the E-value of that agent
milliequivalents (mEq)
expresses concentration of electrolytes in a solution
Note: mEq of cation = mEq of anion = mEq of chemical compound
How do you calculate valence?
Count the cation’s charge
NaCl has a valence of 1 because Na+ is +1
CaCl2 has a valence of 2 because Ca2+ is +2
MgSO4 has a valence of 2 because Mg2+ is +2
K2HPO4 has a valence of 2 because 2 K+ is +2
converting between milligrams and microequivalents
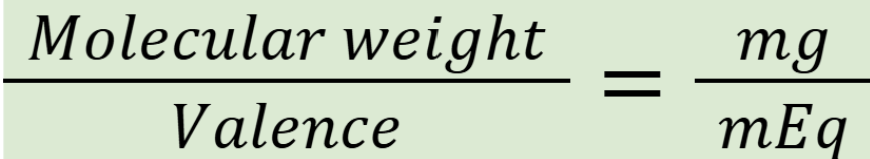
converting between milligrams and millimoles
Using MW as a medium
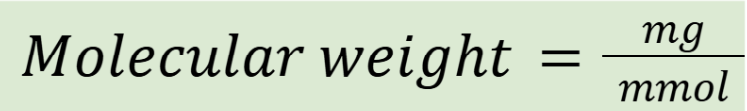
converting between micrograms and micromoles
Using MW as a medium

osmolarity (osmotic pressure)
Proportional to?
concentration of a solution expressed as the total number of solute particles per liter
Proportional to the total number of particles in a solution
For non-electrolytes, total number of particles = 1
For electrolytes, total number of particles depends on the degree of dissociation (e.g., NaCl dissociates into 2 ions, so 1 mmol = 2 mOsmol)
isotonic solution
two solutions consisting of the same osmotic pressure
hypotonic solution
solutions have a lower osmotic pressure (lower concentration of dissolved solutes) than another solution or body fluid
Water will move into the blood cell to maintain homeostasis, causing it to swell
hypertonic solution
solutions that have a higher osmotic pressure (greater concentration of dissolved solutes) than another solution or body fluid
Water will move out of the blood cell to maintain homeostasis, causing it to shrink
osmolarity (milliosmolar concentration) of each component of an admixture
Total milliosmolar concentration of an IV solution is the sum of the milliosmolar concentration of the individual components

principles of measuring volume
Capacity has to be equal to or greater than the volume to be measured
Narrower chamber = More accurate
Least measurable = 20% of the instrument’s capacity
principles of measuring weight
Capacity: maximum weight measurable
Readability: smallest fraction that the balance can be read
Sensitivity requirement (SR): load (weight applied to balance) that will cause change of 1 division
least weighable quantity (LWQ)
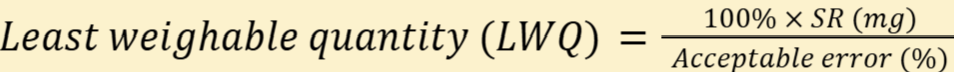
percent error
Note: SR can be substituted for the numerator
Desired amount: amount you’re hoping to see; the most accurate or precise amount
Measured amount: amount you got; the least accurate amount
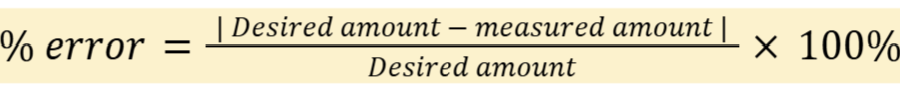
aliquot
portion of a larger whole
Used when we cannot accurately measure a desired amount because it’s too small (i.e., beyond the least amount measurable or the instrument’s capabilities)
What you can do is create a dilution to get a larger volume to measure out and then pull off some to be your aliquot containing the amount you desire in it
Example: 5-mL portion can be an aliquot of a 125-mL dilution
process for finding volume aliquot
Determine the smallest amount measurable (Capacity * 0.2)
Choose the amount of drug to be measured
Choose the amount of diluent to be measured
Calculate the total amount of drug-diluent mixture
Calculate the aliquot amount that contains the desired quantity of drug
Checklist:
Measured drug amount > LWQ
Measured diluent amount > LWQ
Measured drug + diluent amount < Capacity
Aliquot > LWQ
Everything can be measured using at least the smallest increment for measurement
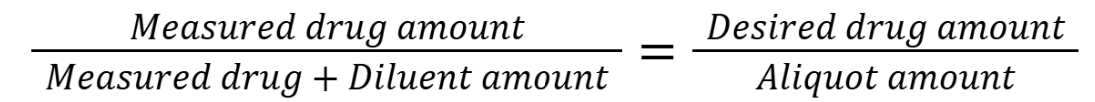
process for finding weight aliquot
Determine the smallest amount measurable (LWQ will either be given or need to be calculated)
Choose the amount of drug to be measured
Choose the amount of diluent to be measured
Calculate the total amount of the drug-diluent mixture
Calculate the aliquot amount that contains the desired quantity of drug
Checklist:
Measured drug amount > LWQ
Measured diluent amount > LWQ
Measured drug + diluent amount < Capacity
Aliquot > LWQ
Everything can be measured using at least the smallest increment for measurement
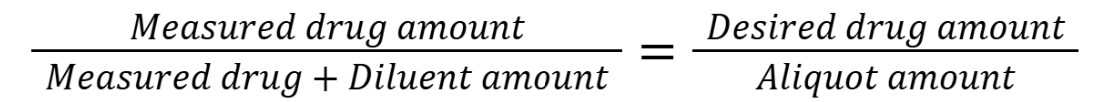
solving by the factor method

steps to determine new concentration of a suspension
Find total amount of amoxicillin in bottle (proportion with medication strength and volume when reconstituted)
Find space occupied by powder (total expected volume - amount supposed to be added)
Find new total volume (amount added by technician + space occupied by powder)
Find new concentration of reconstituted suspension (Step 1 divided by Step 3)
IV infusion
sterile, aqueous preparation administered via IV
Used mostly to provide medications, electrolytes, nutrition, or fluids
Example question: A pharmacist needs to prepare one liter of dextrose 20% solution in sterile water for injection using a 700 mg/mL dextrose injection. How many milliliters of the injection are required? Round to the nearest tenth.
IV push (IVP)
small volume of an IV infusion injected directly into a vein over 1-5 minutes typically
Can also be referred to as a “bolus” dose
Example question: A physician orders midazolam 4 mg IV push STAT. A pharmacist delivers a vial containing midazolam 5 mg/mL. How many milliliters of midazolam should be administered to the patient?
IV admixture
addition of one or more additives to an LVP
Example question: A medication order for a patient who weighs 145 lb calls for amphotericin B 0.25 mg/kg to be added to 500 mL of a 5% dextrose solution. If the amphotericin B is to be obtained from a constituted injection that contains 50 mg/10 mL, how many milliliters should be added to the 5% dextrose solution? Round to the nearest tenth.
flow rate
amount of drug/volume in an IV to be administered over a specific time
Example question: If 20 mg of a drug is added to 500 mL of a LVP, what should the flow rate be in mL/hr in order to deliver 1 mg of drug per hour?
body mass index
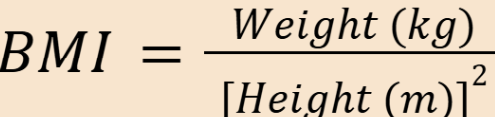
shortcut for ABW and IBW comparison
ABW/IBW * 100 can help get the right interpretation from the table
Carbohydrates = _____ kcal/g
Includes dextrose
Protein = _____ kcal/g
Lipids alone = _____ kcal/g
Lipid emulsion 10% (in carbohydrate base) = _____ kcal/g
Lipid emulsion 20% (in carbohydrate base) = _____ kcal/g
3.4
4
9
11
10