Cell Respiration in DP IB Biology: HL Overview
1/214
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
215 Terms
Adenosine Triphosphate (ATP)
A molecule that provides a short-term store of chemical energy that cells can use to do work.
Energy release in cell respiration
Energy released during the reactions of respiration is transferred to ATP.
Heat loss in respiration
Heat is lost at each step of respiration, which is used to regulate body temperature in endotherms.
Universal energy currency
ATP is described as a universal energy currency because it is used in all organisms.
Hydrolysis of ATP
The hydrolysis of ATP can be carried out quickly and easily wherever energy is required within the cell by the action of ATPase.
Energy release from ATP hydrolysis
A useful quantity of energy is released from the hydrolysis of one ATP molecule, reducing waste and giving the cell control over processes.
Stability of ATP
ATP is relatively stable at cellular pH levels.
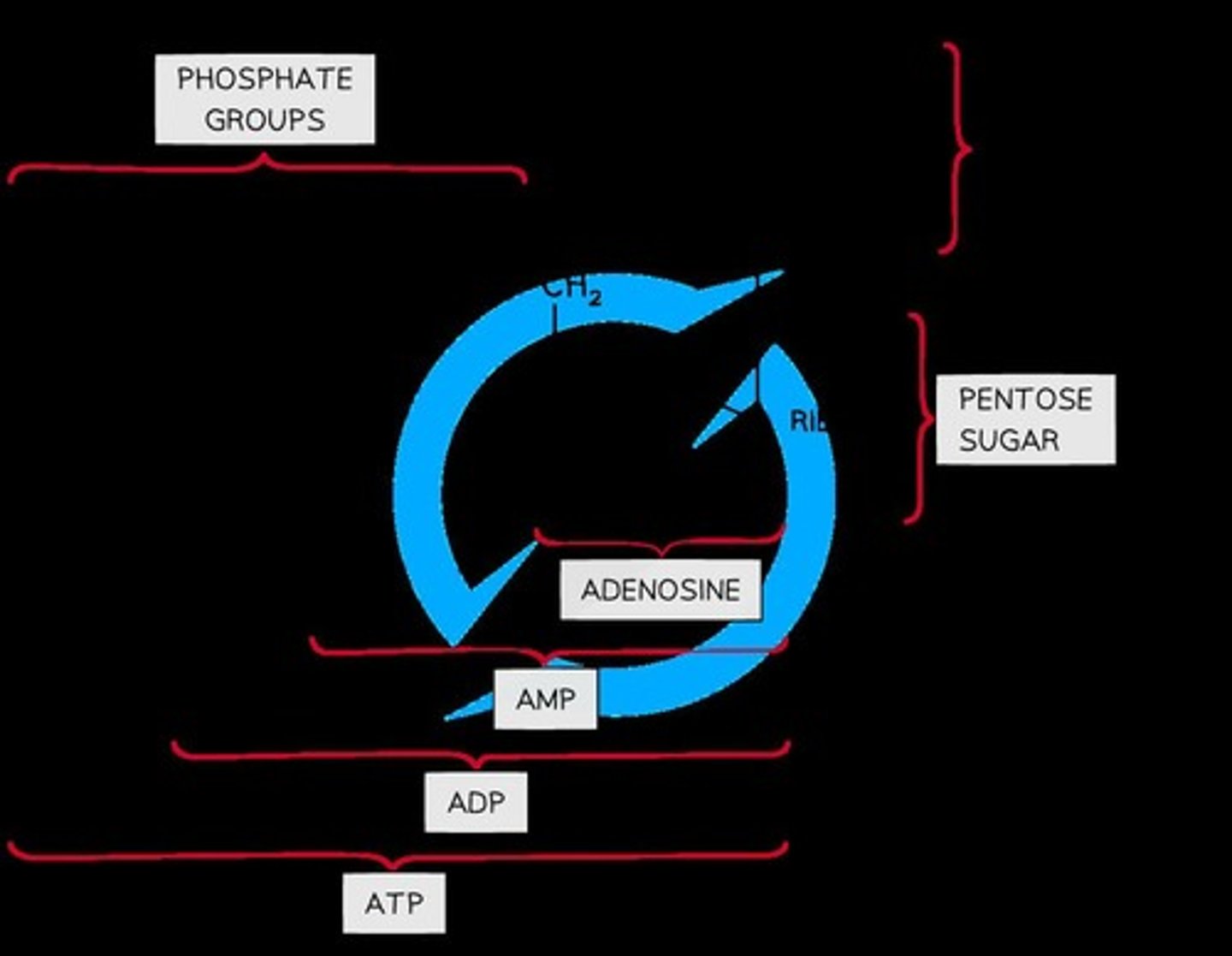
Structure of ATP
ATP is a phosphorylated nucleotide made up of ribose sugar, an adenine base, and three phosphate groups.
Recyclability of ATP
The breakdown of ATP is a reversible reaction; ATP can be reformed from ADP and a phosphate ion.
Quick hydrolysis of ATP
Hydrolysis of ATP is quick and easy, allowing cells to respond to a sudden increase in energy demand.
Solubility of ATP
ATP is soluble and moves easily within cells, allowing it to transport energy to different areas.
Phosphorylated intermediates
ATP forms phosphorylated intermediates, making metabolites more reactive and lowering the activation energy required for a reaction.
Life processes reliant on ATP
Life processes that rely on ATP include anabolic reactions, active transport, cell movement, and movement of cell components.
Conversion of ATP
ATP is readily converted to adenosine diphosphate (ADP) and a phosphate ion (P i), during which energy is released.
Storage of energy
ATP is not stored in living organisms; glucose and fatty acids are used for short-term energy storage, while glycogen, starch, and triglycerides serve as long-term storage.
ATP
A very reactive molecule that is readily converted to ADP and phosphate when releasing its energy.
ADP
A molecule that can be re-converted to ATP during respiration.
ATP Cycle
The constant cycling of ATP and ADP + P within a cell.
Hydrolysis of ATP
The process when ATP is hydrolysed (broken down), producing ADP and phosphate.
Free Energy Release
As ADP forms, free energy is released that can be used for processes within a cell, e.g., DNA synthesis.
Energy Release from ATP
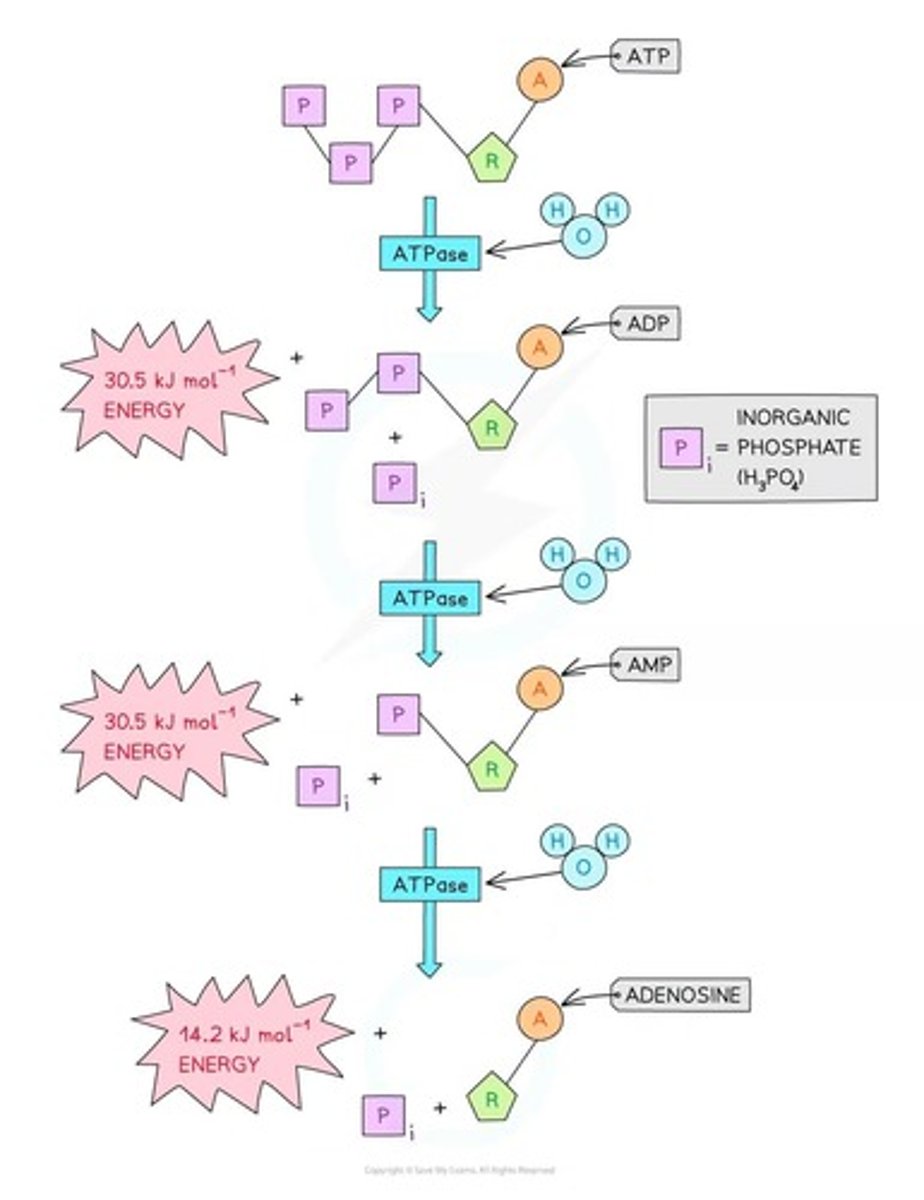
Removal of one phosphate group from ATP releases approximately 30.5 kJ mol⁻¹ of energy, forming ADP.
Energy Release from ADP
Removal of a second phosphate group from ADP also releases approximately 30.5 kJ mol⁻¹ of energy, forming AMP.
Energy Release from AMP
Removal of the third and final phosphate group from AMP releases 14.2 kJ mol⁻¹ of energy, forming adenosine.
ATP Synthesis
ATP is formed when ADP is combined with an inorganic phosphate (Pi) group, which is an energy-requiring reaction.
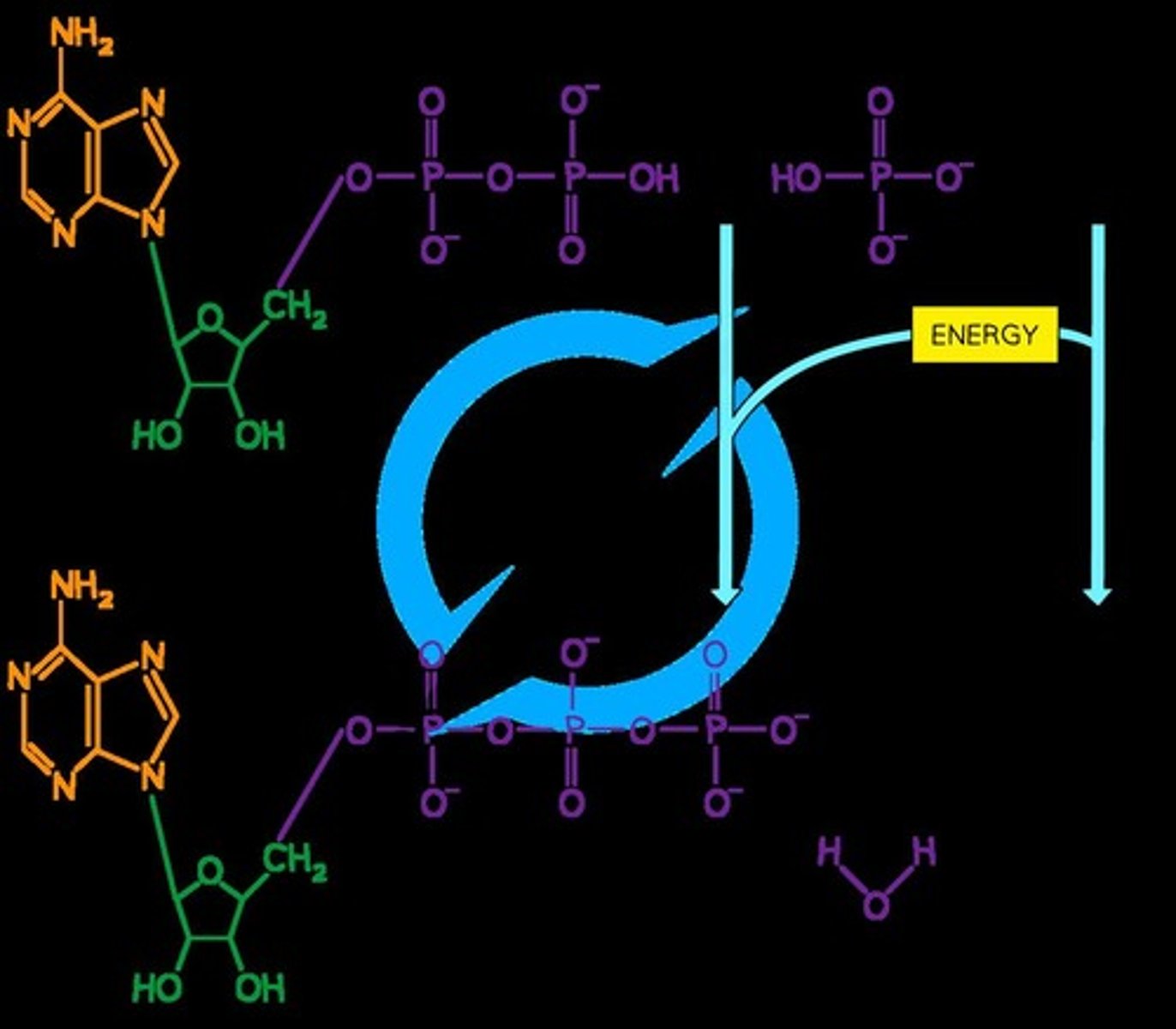
Waste Product of ATP Synthesis
Water is released as a waste product during ATP synthesis, making it a condensation reaction.
Daily ATP Usage
On average, humans use more than 50 kg of ATP in a day but only have a maximum of ~200g of ATP in their body at any given time.
ATP Storage
Organisms cannot build up large stores of ATP, and it rarely passes through the cell surface membrane.
Cell Respiration
A system for producing ATP through the controlled release of energy from organic compounds.
Respiration Process
A series of chemical reactions that happens in every cell to release energy in usable forms from chemical energy stored in food.
Main Respiratory Fuel
Glucose is the main respiratory fuel used in cells.
Use of Lipids and Proteins
Lipids and proteins can also be used as respiratory fuels but must undergo several changes before entering the respiratory pathway.
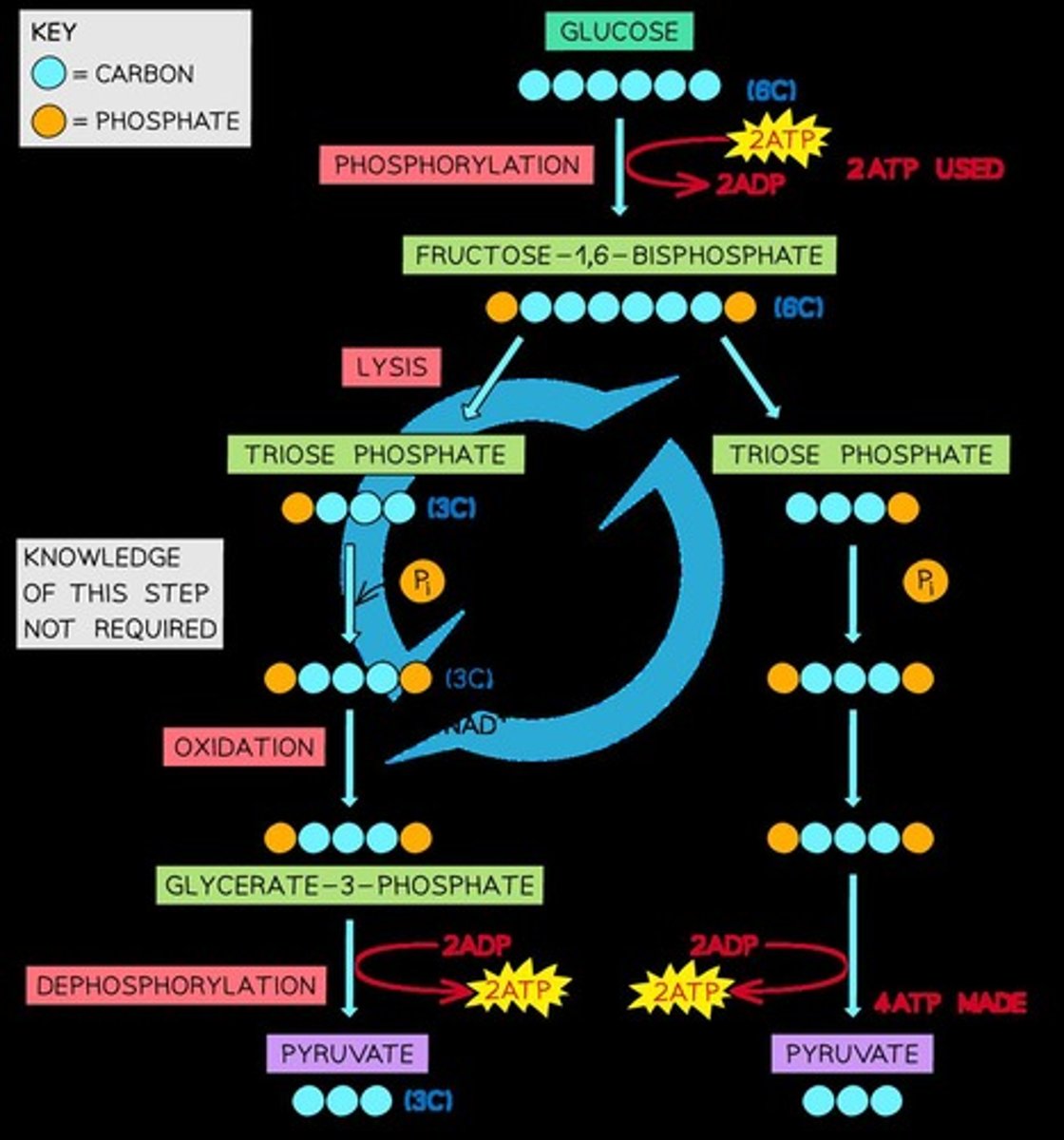
Glycolysis
Glucose can enter glycolysis directly, making it easier to oxidise than lipids and proteins.
Enzymes in Respiration
Enzymes control the release of energy through a series of chemical reactions called a pathway, ending in the production of ATP.
Phosphate Group Linking
To make ATP, a phosphate group is linked to adenosine diphosphate (ADP).
Energy Sources for ATP Production
The energy that is released for ATP production comes from the breakdown of organic molecules.
Uses of Released Energy
The energy released is used for fuelling anabolic processes, muscle contraction, fuelling active transport, moving molecules around the cell, and generating heat.
Respiration
A chemical process that involves the transfer of chemical potential energy from nutrient molecules into a usable energy form (through the synthesis of ATP) that can be used for work within an organism.
Gas Exchange
The exchange of carbon dioxide and oxygen at the alveoli or cells.
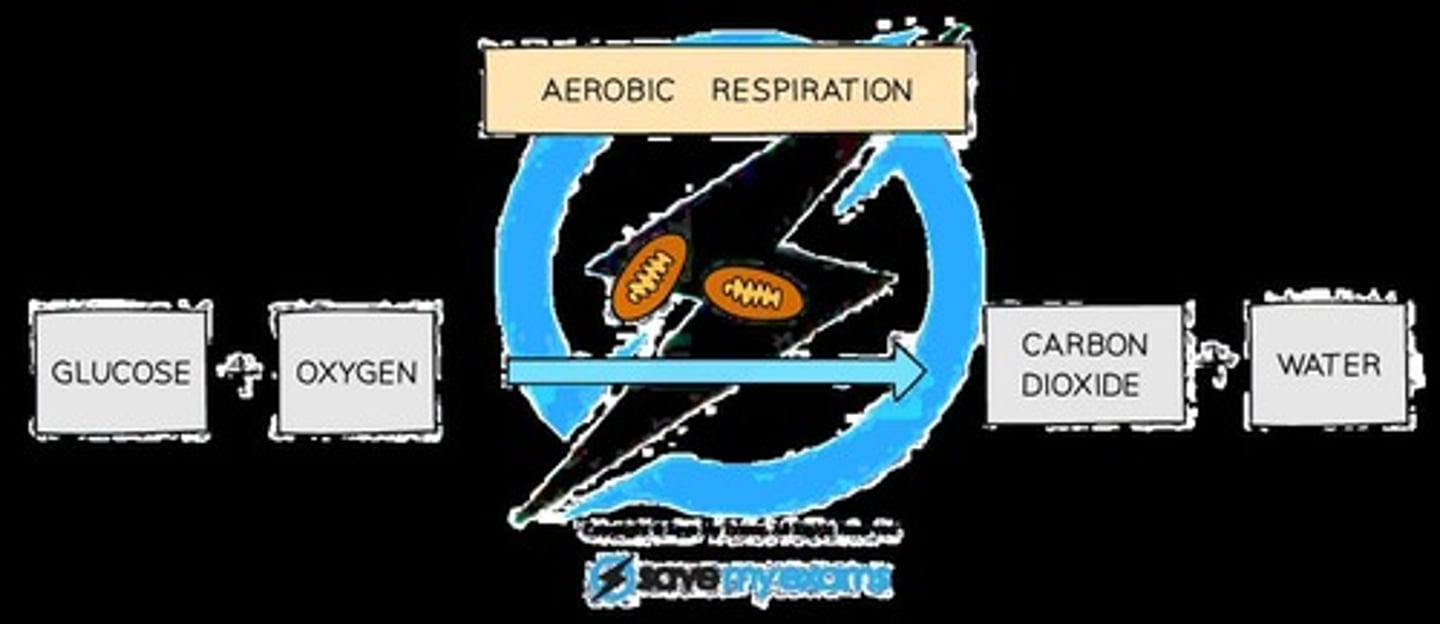
Aerobic Respiration
The process of breaking down a respiratory substrate in order to produce ATP using oxygen.
Anaerobic Respiration
The process that takes place in the absence of oxygen and breaks down a respiratory substrate but produces less ATP for the cell.

Main Respiratory Substrate
Glucose.
ATP Yield in Aerobic Respiration
Approx. 36 ATP molecules per molecule of glucose.
ATP Yield in Anaerobic Respiration
2 ATP molecules per molecule of glucose.
Waste Product of Aerobic Respiration
Carbon dioxide (CO2) which has to be excreted.
By-product of Aerobic Respiration
Water, which contributes to the organism's water needs.
Location of Aerobic Respiration Reactions
Most reactions take place in the mitochondria.
Location of Anaerobic Respiration Reactions
Reactions occur in the cytoplasm of cells and do not involve the mitochondria.
Oxygen Requirement for Aerobic Respiration
Yes, oxygen is required.
Oxygen Requirement for Anaerobic Respiration
No, oxygen is not required.
Oxidation of Glucose in Aerobic Respiration
Complete oxidation.
Oxidation of Glucose in Anaerobic Respiration
Incomplete oxidation.
Products of Aerobic Respiration
Carbon dioxide (CO2) and water (H2O).
Products of Anaerobic Respiration in Animals
Lactate.
Products of Anaerobic Respiration in Plants and Yeasts
Ethanol and carbon dioxide (CO2).
Energy Yield Comparison
Anaerobic respiration yields much lower energy than aerobic respiration.
Short Supply of ATP
Anaerobic respiration can provide a short discharge of energy when oxygen runs out.
Word Equations for Respiration
You should be able to write simple word equations for both types of respiration, with glucose as the substrate.
ATP
Produced during both aerobic and anaerobic respiration.
Rate of Cell Respiration
May vary depending on metabolic activity, organism size, oxygen supply, respiratory substrates, temperature, and pH.
Metabolically Active Cells
Muscle cells have a higher rate of cell respiration than adipose cells due to higher energy needs.
Surface Area: Volume Ratio
Smaller organisms have a higher ratio than larger organisms, leading to a higher rate of respiration to compensate for heat loss.
Oxygen Availability
Low oxygen levels lead cells to respire anaerobically.
Supply of Respiratory Substrates
Glucose availability is crucial as it is the main respiratory substrate; lower substrate supply results in a lower respiration rate.
Temperature
Respiration rate increases up to the optimum temperature of the enzymes, after which it drops due to enzyme denaturation.
pH
Carbon dioxide released during respiration decreases pH, which may denature enzymes involved in respiration.
Respirometers
Used to measure the rate of oxygen consumption during respiration in organisms.
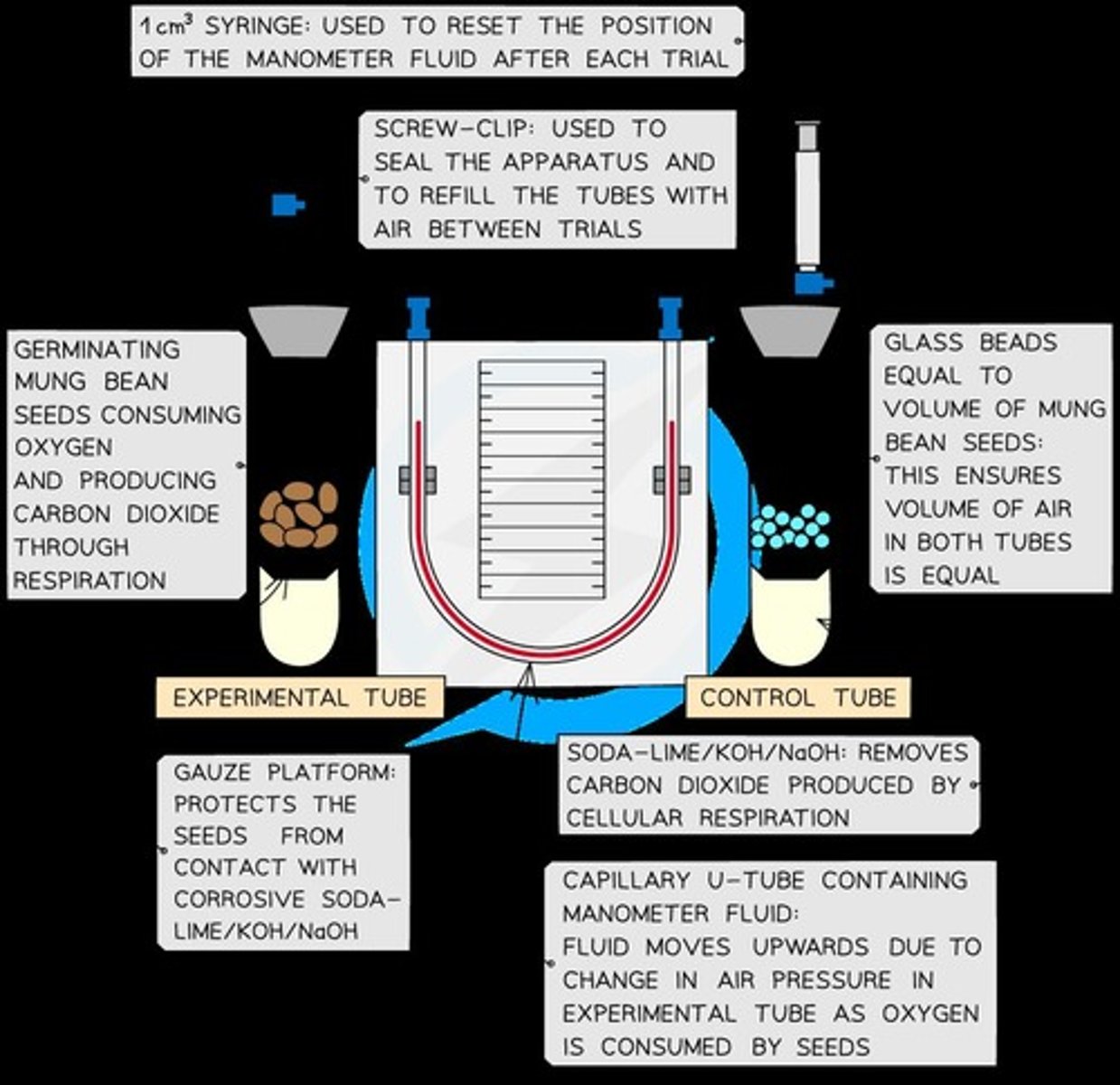
Experimental Organisms
Experiments typically require live organisms such as seeds or invertebrates.
Respirometer Features
Includes a sealed container with live organisms, an alkaline solution to absorb CO2, and a capillary tube against a graduated scale.
Pressure Drop
The CO2 released is absorbed by the alkali, reducing air pressure inside the sealed chamber.
Temperature Control
Respirometers must be kept in temperature-controlled conditions to avoid affecting air pressure.
Repeat Readings
Carried out to identify and eliminate anomalies, providing a reliable mean.
Technology in Respiration Measurement
Oxygen sensors and CO2 monitors measure concentrations in real-time without exposing subjects to hazards.
Dataloggers
Record data over time for later analysis.
Respirometer Setup
Typical setup includes a sealed chamber, alkaline solution, and a capillary tube.
Gas Volume Change Calculation
Volume of oxygen consumed (mm³ min⁻¹) is calculated using the formula: 2πrh, where r is the radius of the capillary tube and h is the distance moved by the manometer fluid.
Average Rate of Respiration
Determined using the volume of oxygen consumed per unit time.
Worked Example
A respirometer with germinating mung beans showed the liquid moved 2.3 cm in 25 minutes.
Average rate of respiration
Measured as the rate of oxygen uptake, calculated in mm min.
Capillary tube internal diameter
0.30 mm.
Cross-sectional area of capillary tube
Calculated using the formula πr², where r = 0.15 mm, resulting in 0.0707 mm².
Volume of oxygen taken up
Calculated as 2πr h, where h = 23 mm, resulting in 1.625 mm³.
Average rate of oxygen consumption per minute
Calculated as 1.625 ÷ 25, resulting in 0.065 mm³ min⁻¹.
Oxidation
The loss of electrons.
Reduction
The gain of electrons.
Redox reactions
Reactions that involve the transfer of electrons between molecules.
Exergonic reaction
A reaction that releases energy to the surroundings.
Endergonic reaction
A reaction that absorbs energy from the surroundings.
Reducing agents
Molecules that have a strong tendency to lose/donate their electrons.
Oxidising agents
Molecules that have a strong tendency to gain electrons.
NAD
Nicotinamide adenine dinucleotide, the primary electron carrier involved in respiration.
FAD
Flavin adenine dinucleotide, another electron carrier used in respiration.
Coenzymes
Molecules that serve as links between redox reactions.
Reduced NAD
The form of NAD after gaining electrons and hydrogen ions, represented as NADH.
Reduced FAD
The form of FAD after gaining electrons and hydrogen ions, represented as FADH₂.
OILRIG
A mnemonic to remember that Oxidation Is Loss and Reduction Is Gain.
Oxidised form of NAD
NAD⁺, which acts as an oxidising agent.
Reduced form of NAD
NADH, which acts as a reducing agent.
Electron carriers
Molecules used to transport electrons gained in redox reactions.
Return to original form
When electron carriers lose electrons, they revert to their original form.
Glycolysis
The first stage of respiration that takes place in the cytoplasm of the cell.