Lecture 15. Lecture 14 was going through questions
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
Summarise properties of benzene
Each carbon contains a pi orbital containing one electron. This creates a pi cloud above and below planar ring of carbons. This means conjugation can take place which leads to benzene’s increased stability - 152 kJ resonance stabilization energy. All the bond lengths are equal but longer than the general C=C bond but shorter than the general C-C bond.
Explain conjugation
You see conjugation where you have alternating single and double bonds or you have a pi bond next to an tom with a lone pair or an empty pi orbital. This allows the pi orbitals to overlap and the electrons delocalizes across a larger area, which is a stabilizing effect.
When reacting with benzene, what must we do to the reactant
Benzene reacts with electrophiles but only very reactive ones. Therefore, reactions must take place to create very reactive species
Why can an ester be both electron donating and withdrawing?
It depends on which side the ester group is bonded to the benzene ring. If the the ring is attached to the carbonyl, then the ester group is electron withdrawing. If the ring is attached to the electronegative oxygen, the ester ester becomes electron donating.
What’s special about when a halogen is attached to benzene ring?
A halogen is inductively electron withdrawing but because it have lone pairs, it’s mesomerically electron donating. Usually the mesomeric effects wins out over the inductive effects but not for halogens. Due to them being so electronegative, their inductive effects are much stronger.
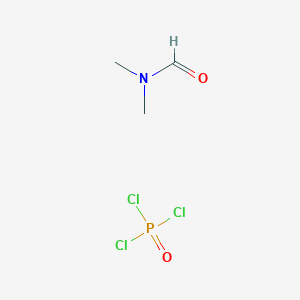
Vilsmeier-Haack formylation - summarise and what are the reactants?
We are trying to add a formyl group to a benzene ring. To create the electrophile, we use DMF and phosphorus oxychloride. An iminium ion is formed and we then use water to convert to our formyl group.
What happens when we want to substitute a group already attached to benzene out
Usually a proton is substituted out but we can substitute other groups as long they form a stable cation. A common example is the removal of the tertiary alkyl group. Using H+ from a strong acid, you can remove it as we know the alkyl group is very stable as a carbocation.
