PHS 2201- Chapter 1
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Central Dogma of Biochemistry
1) Inherited information can be stored in cells and transferred to offspring using biological molecules/changes in the stored information can be altered to introduce variability within species but also cause disease.
2) Provide the structure by which cells and organisms are built.
3) Carry out functions important for life.
mRNA —> DNA —> Protein
Spontaneous Generation
Living organisms arise from nothingness.
1860’s – Demonstrated that bacteria exist in air (Louis Pasteur)
Led to Cell Theory
Vitalism
Living systems do not obey the same chemical principles as inert materials
Challenged by Wohler in 1828 with discovery that urea can be synthesized from ammonia and bicarbonate.
In 1897 Eduard and Hans Buchner demonstrated fermentation could exist in an extract from ruptured cells.
Collision Theory
Rate of reaction is proportional to the number of collisions
Concentration of reactant directly proportional to collision chance.
Forward Reaction Rates
Rate of substrates conversion to products
A + B → C + D
ratef = kf [A][B]
Reverse Reaction Rates
Rate of products returning to substrates
C + D → A + B
rater = kr [C][D]
Rate Constant
Forward Rate = kf
Reverse Rate = kr
Constant of proportionality between rate and concentration.
Equilibrium
Forward and reverse reactions equal each other
ratef = rater
kf [A][B] = kr [C][D]
kf / kr = [C][D] / [A][B] = Keq
While a single reaction may exist in equilibrium, so may multiple reactions.
Relates kinetics (rate process tied to a time element) to equilibrium (independent of time)
The Steady State
Living cells are never actually at equilibrium
Need substrate (S), intermediate (I), and product (P)
S → I → P
S → I → J → K →L → P: Can be better suited to describe what looks to be equilibrium
Thermodynamics (Classical Approach)
Few postulates and definitions
Makes no assumptions about the exact nature of the systems under investigation
Description of the system at near-equilibrium, using properties which are measurable
Consistent set of equations
Thermodynamics (Statistical Approach)
Considers behavior of large collections of molecules
Allows for more mechanistic conclusions
More narrowly applicable
Consistent set of equations
Internal Energy
Energy of the system under study
Sum of the work (energy of motion) and heat of the system
Enthalpy
Heat released or absorbed by a reaction
Entropy
Number of ways energy can be distributed
Increases with an increase in energy dispersion
Free Energy
Combination of enthalpy and energy
Used to determine if a reaction will proceed
∆G = ∆H - T∆S
NADH
Nicotinamide adenine dinucleotide
High energy electrons
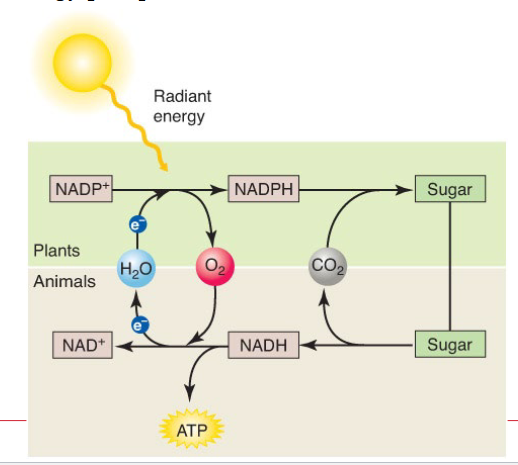
ATP
Adenosine triphosphate
High energy phosphate
Cellular Currency
Cell Theory
Cells are the fundamental unit of living systems
Smallest unit of life
Single Cell Organisms vs. Mammalian Cells
Single Cell: Largest number of species/prokaryotes (i.e., bacteria, yeasts, protozoans)
Mammalian: Multicellular organisms
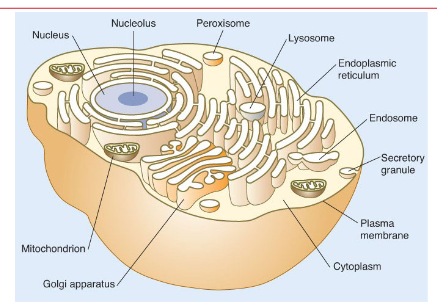
Internal organelles (mitochondria, endoplasmic reticulum, nuclear membrane)
Semipermeable membrane (plasma membrane)
Create separate water spaces to isolate chemical reactions
Need to communicate across these spaces, diffuse, or utilize a specific transporter to cross membrane
Evolution
Charles Darwin’s work in the Galapagos Islands of Ecuador “On the Origin of Species”
Species arose from other species
Those that could adapt best to their environment were able to survive because they lived to reproduce.
Adaptive Characteristics
Traits that best survive a condition stay, those that do not, die out.
Evolution in Biochemistry
Map the formation of enzymes and DNA sequences
Numerous enzymes and DNA sequences diverge from their origin
Classical Hierarchy
Organize the diversity of life
Catalog the complexity of the immense number of known species
3 of 5 kingdoms studied in biochemistry:
Animals – Plants – Prokaryotes
Biological Systems (Holistic View)
View close to top
Widest view possible
Answers questions that are physiologically relevant
Biological Systems (Reductionist View)
Molecular view
Molecular interactions