Nanoparticles
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
What are nanoparticles?
Small particles of around 1-100nm in size.
What are the properties of nanoparticles?
They are smaller than the wavelength of visible light, therefore cannot be seen in optical microscopes.
They are very light and therefore dont precipitate from suspensions and form colloidal solutions instead.
Their properties are intermediate between molecules and bulk materials, some properties depend on nanoparticle size.
What are some everyday applications of nanoparticles?
Hard drives- use magnetic properties of NPs.
Sunscreens- use optical properties (TiO2 pigments that scatter UV)
Self cleaning windows- photocatalyst TiO2 oxidises dirt particles
Coloured glass
What are the two ways of preparing nanoparticles?
Bottom-up: ‘precipitation’, self assembly of molecules into NPs.
Top-down: ‘milling’, break down of bulk material into NPs.
What are the stages of nanoparticle formation (precipitation)?
Nucleation
Growth
Ostwald ripening
Agglomeration/stabilisation
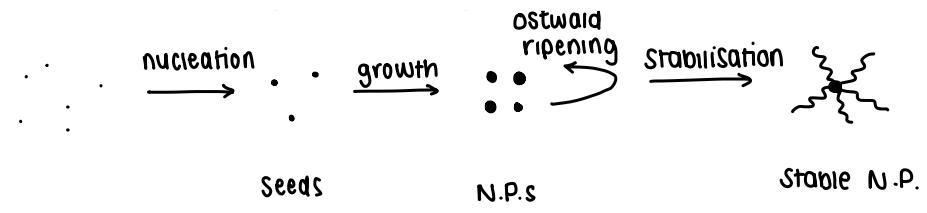
What is nucleation?
The formation of tiny seeds from which a NP can grow from.
What happens in the growth of nanoparticles?
The seeds grow into a NP- this occurs quite fast and all seeds grow at the same rate.
What is Ostwald ripening?
When bigger particles grow at the expense of smaller ones, usually occurs slowly.
The driving force of this is the reduction of SA, high T is needed.
What is agglomeration?
The stabilisation of growing NPs to stop them from growing further (until they are no longer classed as NPs).
Molecular stabilisers are used which bind to the surface and stop further growth.
There are 2 types: electrostatic and steric.
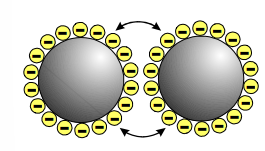
How does electrostatic stabilisation work?
A layer of counter ions adsorb onto the surface around the NPs, making them negatively charged. This makes the NPs repel, so they cannot combine to grow further.
This only works in water and at low concentrations of salt.
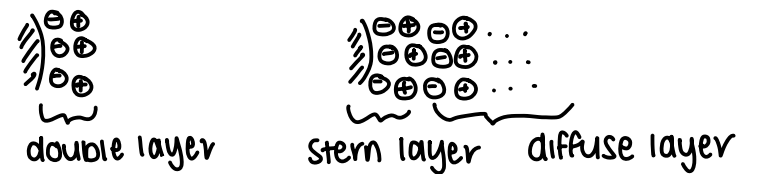
What is the double layer theory of electrostatic stabilisation?
A layer of counter ions forms around the NP.
Helmholtz suggests there is a double layer.
The Gouy-Chapman-Stern model suggests there are multiple layers. This consists of a Stern layer closest to the NP surface and a diffuse layer.
How does steric stabilisation work?
The NP is coated with bulky stabilisers, causing the NPs to sterically repulse one another so they can no longer grow.
What makes a good steric stabiliser?
Long chain bulky groups are the best, they need to adsorb onto the NP strongly.
The stabiliser must also be chemically inert to prevent reactions.
The terminal group determines solubility.

What is monodispersity?
When all molecules are the same size.
How do you achieve monodisperse NPs?
They require short nucleation and slow growth.
Small NPs require fast stabilisation, this is achieved by increasing stabiliser conc.
What is template directed assembly?
Another method for bottom-up NP formation.
Involves using a nanocontainer which contains the seeds and is eventually filled up by the NP.
Biological cages, inorganic nanotubes and colloidal systems can be used as nanocontainers.
NP size is controlled by size of container.

How does nucleation of metal nanoparticles occur via reduction?
The metal cation is reduced (NaBH4 is common) and then grown into a NP.
A fast reducing agent leads to fast nucleation and growth, therefore giving small and polydisperse NP.
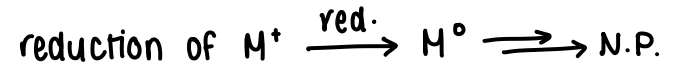
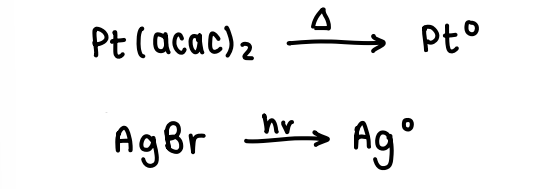
How does nucleation of metal nanoparticles occur via decomposition of organometallic precursors?
Carbonyl complexes or metal halides are often used, they are heated to form the metal NPs or can sometimes be reduced photochemically.
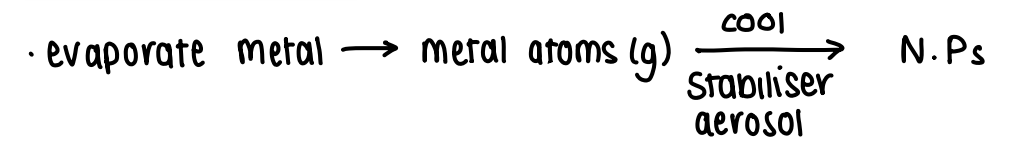
How does nucleation of metal nanoparticles occur via rapid cooling of metal vapour?
The metal is evaporated, forming gaseous metal atoms. These are then cooled with a stabiliser aerosol to form NPs.
How can you stabilise metal nanoparticles?
Can use different ligands:
Thiols (RSH), they are soft ligands good for noble metals. Long alkyl chains are good for steric stabilisation.
Phosphines (R3P), they are soft ligands.
Amines, they are harder ligands and good for Cu
Acids (RCOOH, RPO3H), they are hard ligands and good for harder metals such as Fe.
Halide ions can be used for electrostatic stabilisation, bulky counter ions can then be added for steric stabilisation
Citrates
Polymers (PVP, PVA) are good stabilisers due to the chelate effect and bind weakly.
How do you control size of metal nanoparticles?
Using high T methods gives a better control over the rate of reaction.
High stabiliser conc gives smaller particles, the nature of the stabiliser is also important as fast stabilisers (e.g. RSH) give smaller particles. Slower stabilisers (e.g. R3N) give bigger particles.
What are metal alloy nanoparticles?
There are 2 types:
A homogeneous alloy of metals that combine to form a NP.
A core and shell structure formed in which one type of metal forms a core and another forms a shell around it.
How do you form homogenous metal alloy nanoparticles?
Mix 2 metal precursors.
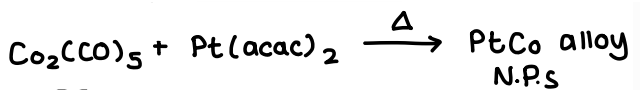
How do you form core shell metal alloy nanoparticles?
Make one metal NP and use this as a seed for the growth of a shell.
What are metal oxide nanoparticles and how are they made?
MxOy structure.
They are made through oxidation of metal NPs, hydrolysis of metal salts (this is fast, so gives poor monodispersity) or high T decomposition of precursors.
They can be stabilised by RCOOH or polymers.
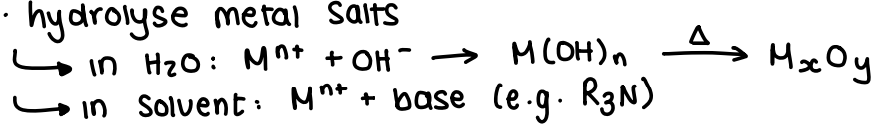
What are metal chalcogenide nanoparticles and how are they formed?
Metal NPs that contain S2- or Se2-
Formed through fast reactions at room T (giving polydisperse NPs) or thermal decomposition (good monodispersity).
Thermal decomposition requires the precursor to be injected at high T (giving fast nucleation), then remove the heat so that growth occurs slowly and then Oswalt ripening leads to bigger NPs. The stabiliser (R3P/R3PO and R3N) can be used as solvent.
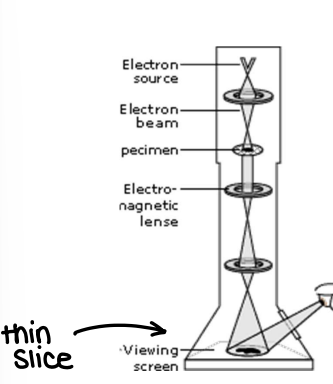
How is TEM used to characterise nanoparticles?
Shines electrons on a thin slice of the NP, giving a high res 2D image, even allowing view of atoms.
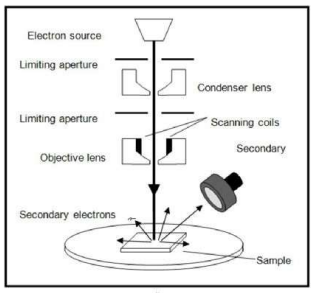
How is SEM used to characterise nanoparticles?
Electrons shone onto a sample of nanoparticles giving a 3D image of the nanoparticles, however resolution is poorer.
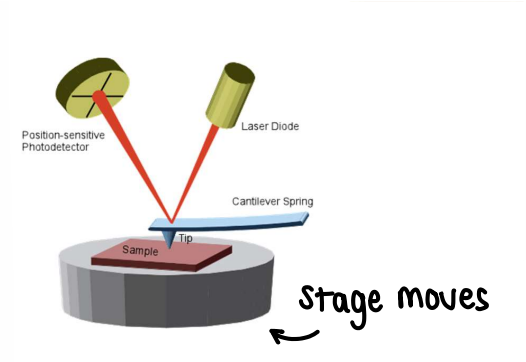
How is AFM used to characterise nanoparticles?
AFM (Atomic Force Microscopy) shines a laser onto a sample, giving an image of the nanoparticles. The resolution can range from atomic to micrometers. It gives you info on the height of the sample.
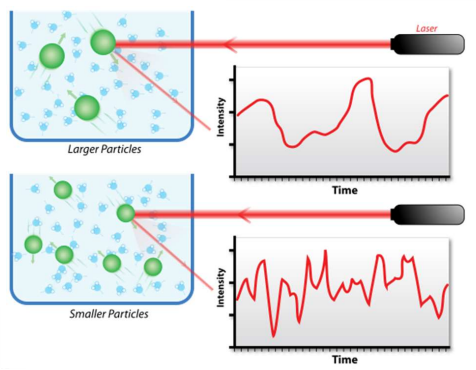
How is DLS used to characterise nanoparticles?
DLS (Dynamic Light Scattering) shines light onto NPs, causing it to be scattered and creates an intensity v time graph. As the NPs rotate, light intensity fluctuates- slow rotation means less fluctuation. This means you can find the size of NPs as small NPs tumble quickly, whereas larger ones tumble slower.
However it is harder to analyse if the NPs are not spherical.
How is NMR used to characterise nanoparticles?
The spectrum shows the ligands attached to the nanoparticle. However, this requires NPs to tumble quickly.
The groups closest to the core move slowly in solution so have little/no NMR signal, whereas groups further away tumble quickly so have larger broader peaks.
How do you purify nanoparticles?
Using techniques that rely on NP size (must be soluble NPs):
Centrifugation, works for even heavy NPs.
Dialysis (through a semipermeable membrane), the NPs are retained and small molecules are able to pass through.
GPC (Gel Permeation Chromatography.
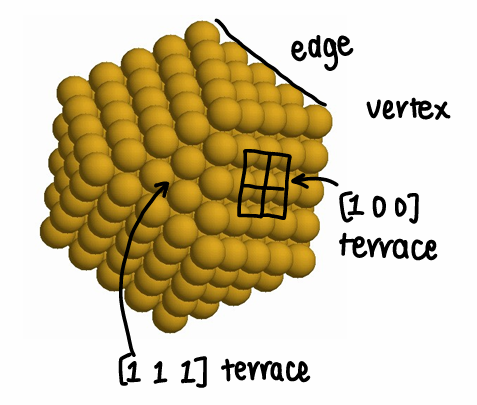
How does nanoparticle shape affect properties?
NPs are not spheres- they can have defects which determine their chemical properties, however this makes them difficult to control and study.
There are binding sites on NPs that can vary- the NP properties depend strongly on the nature of the site.
What are nanorods?
Nanoparticles shaped like rods- these can be beneficial in some applications.
Their synthesis is template directed, some stabilisers selectively bind to a particular face, promoting growth on other faces (desymmetrisation).
What are plasmons?
Surface electrons (metals only).
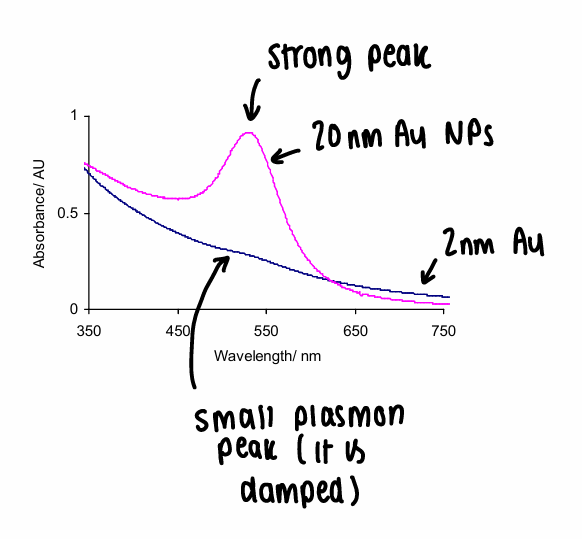
What causes a plasmon peak to be seen in UV spec?
The absorption of light can cause a collective oscillation of plasmons on the NP, causing an inverse of the electrons- this only occurs at specific wavelengths.
For some metals, the plasmon peak is in the visible region, therefore determining the NPs colour (changing NP size changes the colour).
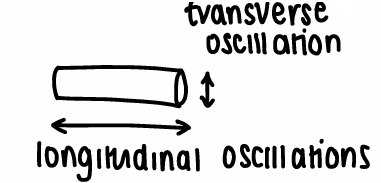
What do plasmon peaks look like for nanorods and why?
The nanorods can have transverse and longitudinal oscillations, causing there to be two plasmon peaks.
The transverse oscillation is similar to that of a sphere, whereas longitudinal oscillations depend on the length of the rod.
How can plasmons be used in sensing applications?
An aggregation of NPs causes plasmon-plasmon interactions. This causes a wavelength shift from around 500nm to around 800nm, therefore changing the colour.
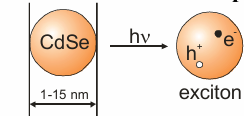
What are quantum dots?
They are semiconductor nanoparticles that can fluoresce.
When excited, the quantum dot forms an electron hole pair. Recombination of the electron and hole leads to fluorescence.
They have important applications as fluorescent labels in biology.
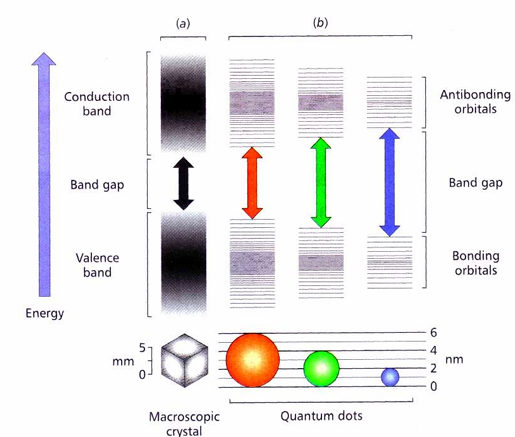
How do quantum dots work?
In a bulk semiconductor, AOs overlap to form bands. However, in NPs there are fewer AOs, the smaller the particle, the fewer AOs.
As the overlap becomes lesser, the band gap between orbitals increases. The different energies of this band gap is what leads to a different fluorescent colour.
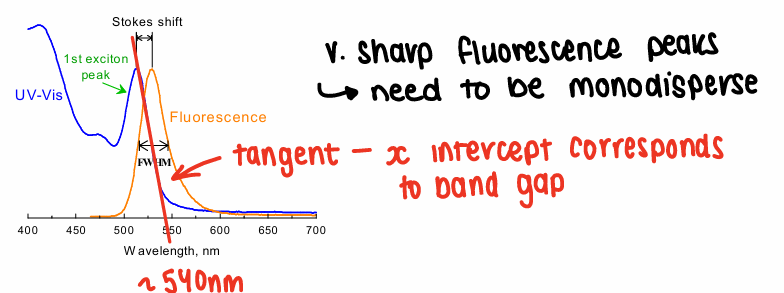
How do you find the band gap energy?
Using a graph of the fluorescence wavelength, draw a tangent on the graph. The x intercept corresponds to the band gap energy.
E = hv = h(c/wavelength)
Units for band gap E is often in eV.
The peaks need to be sharp, therefore the NP needs to be monodisperse.
What are the advantages of quantum dots?
Tuneable in colour
Used for multicolour labelling
All colours can be excited with a single wavelength
They are resistant to photobleaching
What are the disadvantages of quantum dots:
They are quite large.
They are prone to aggregation
They are quite toxic (can lead Cd2+)
They ‘blink’- their colour may become invisible.
How are TiO2 nanoparticles used as sunscreen?
TiO2 is a semiconductor with a bandgap in the UV region.
The NP is transparent to visible light, but UV light is absorbed to excite electrons across the band gap.
Why are nanoparticles used for catalysis?
NPs can be soluble.
They have a high proportion of surface area which is desired for a catalyst, they therefore have a large number of catalytically active unsaturated sites.
They have size dependent properties, however activity is often determined by defects.
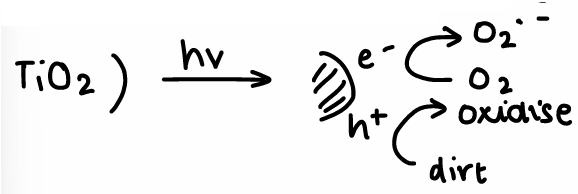
How are nanoparticles used for photocatalysis?
Semiconductor NPs have electron-hole pairs which can diffuse to NP surface and initiate radical reactions.
E.g. TiO2, it has a non specific oxidation of organic contaminants.
How are nanoparticles used in MRI?
MRI uses contrast agents to improve sensitivity (must be paramagnetic).
NPs like Fe3O4 are used.
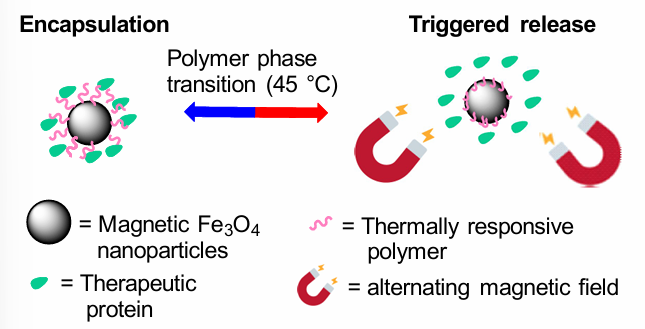
How are nanoparticles used in drug delivery?
Therapeutic proteins can be attached to NPs through thermally responsive polymers, which then phase transition when exposed to specific Ts, releasing the protein.