3.1.3.5 shapes of molecules
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
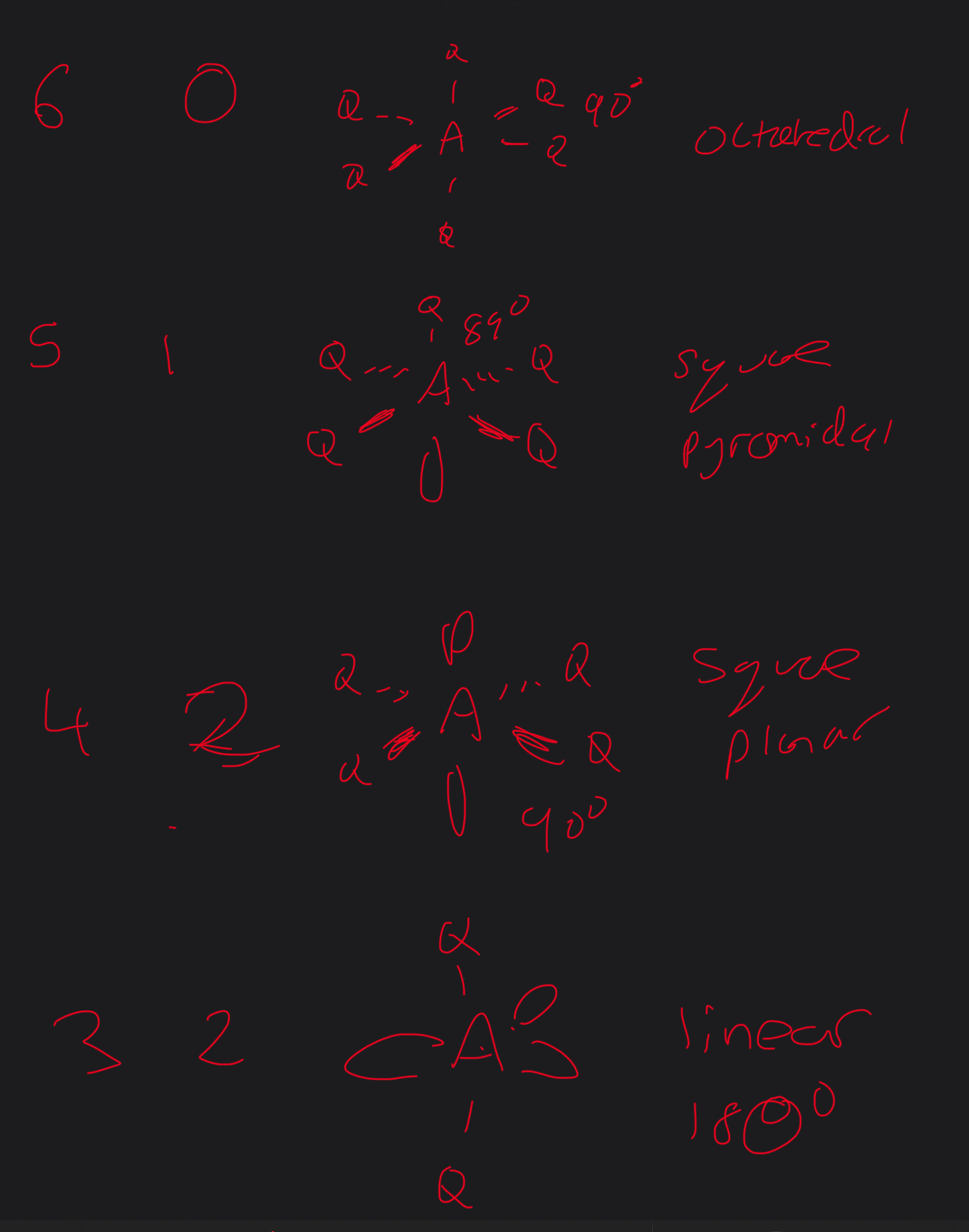
What are the 2 types of pairs
Bonding
Lone
The pairs will ——
Repel each other
What is the shape of any molecule or ion a consequence
Number of electrons pairs which repel each other as far as possible
What repels more lone. Pairs or bond pairs
Lone pairs repel more which then reduces the bod angles to a small extent
What is the order of repulsion of the bonds

What is the explanation par of Bond angles for linear
2bp of electrons repel equally and the molecule takes this shape to minimise repulsion
What are all the main shapes called and what are the bond angles

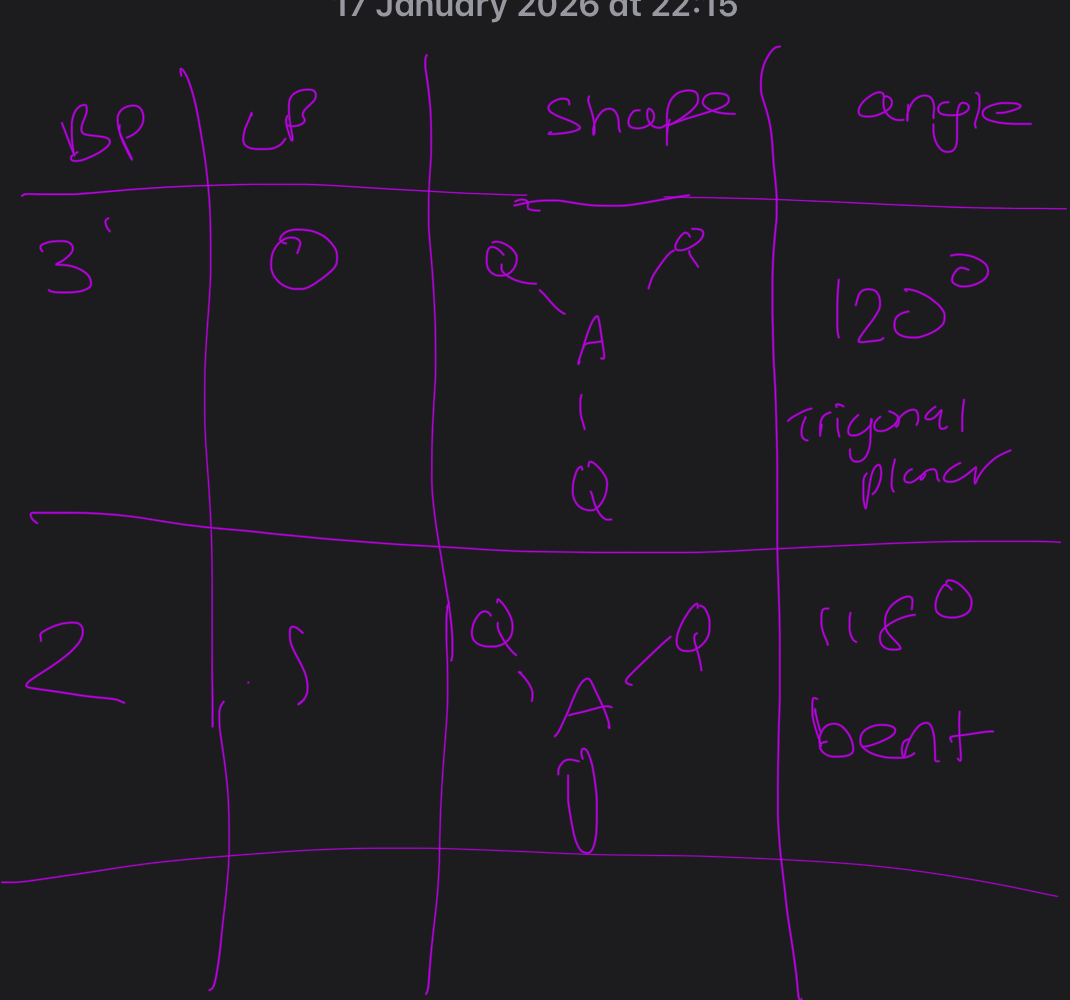
What ae alll the different shapes for a total number of 3 electron pairs
What about 4 electron pairs
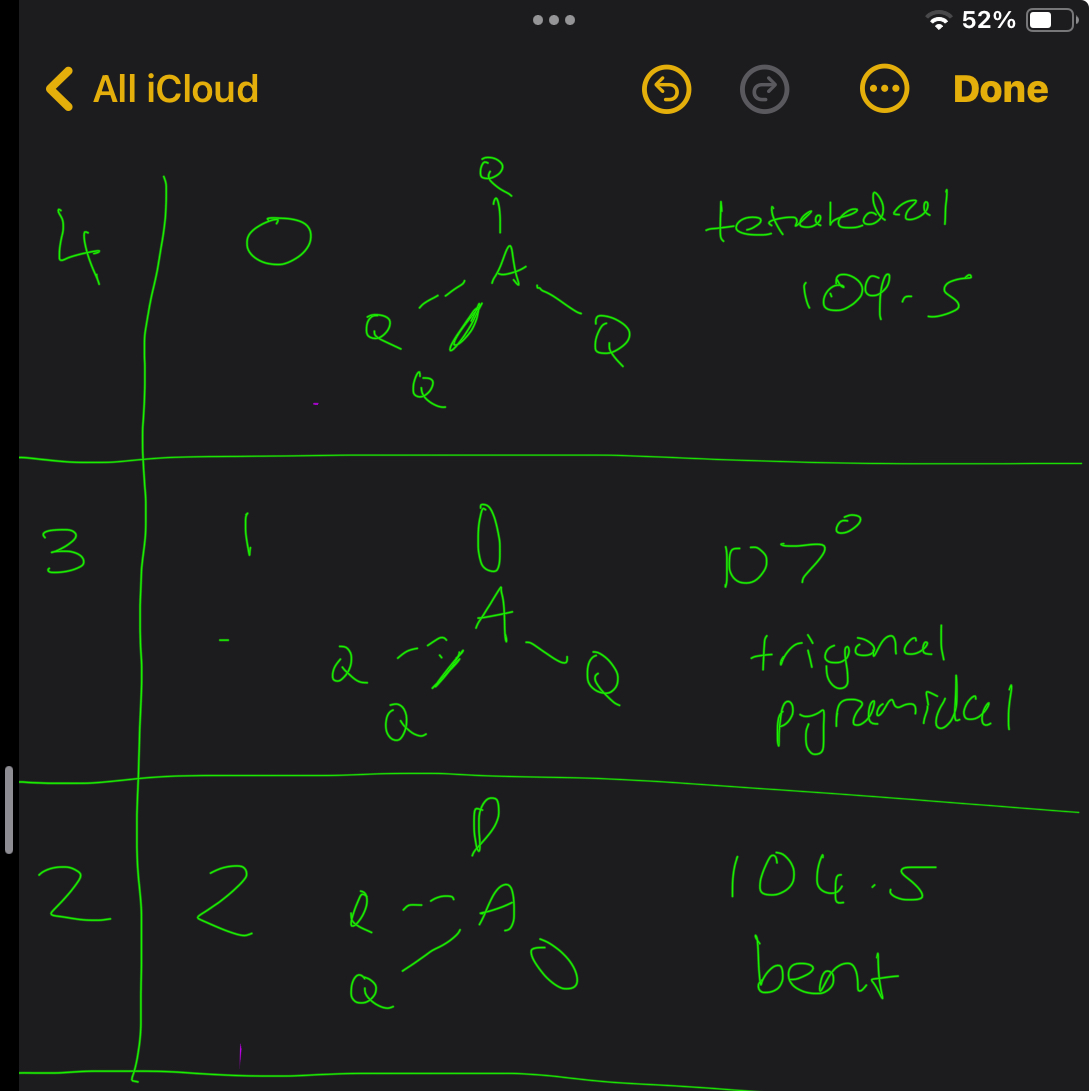
5 electron pairs

6 electrons