Advanced Placement Chemistry: Quiz 3, Chapter 6-8
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
The wave nature of light, electromagnetic radiation (radiant energy), wavelength (λ, lambda), Frequency (ν, nu).

Quantized Energy and Photons, Photo Electric Effect, Hot Objects and the Quantities of Energy, quantum
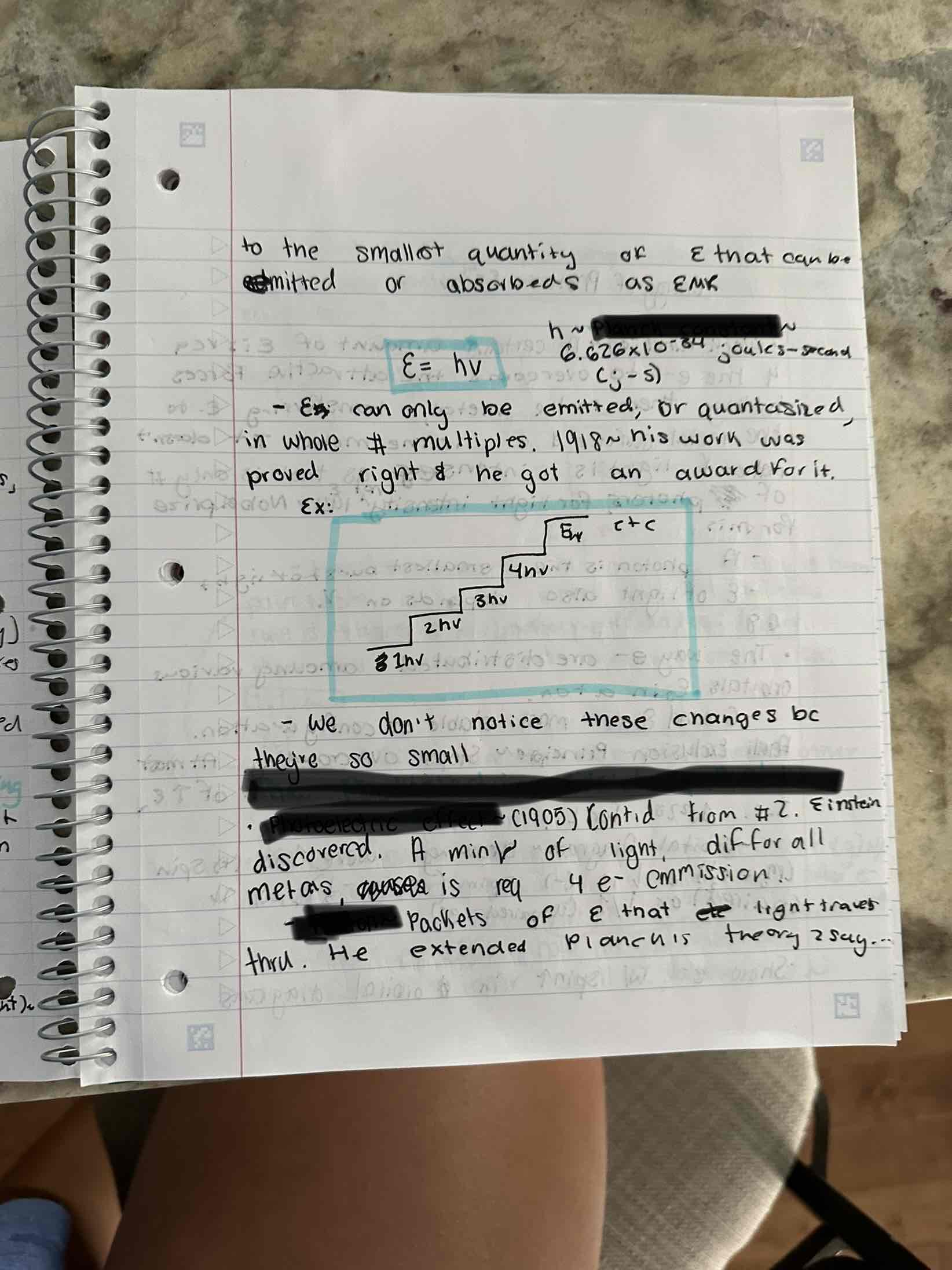
Planch Constant, The Photo Electric Effect and Atoms, Photo Electric Effect, Photon

Electron Configurations, Pauli Exclusion Principle, Orbital Diagram, Hund’s Rule

Alfbau, Hund’s Rule Condesed Electron Configuration, Core Electrons, Valance Electrons

Transition Metals and Elements, The Lanthanides and ACtinides, Lantinide Elements (4f, rare earth elements).

Actinide Elements (5f), electron configurations, representative elements (main group elements), f-block metals, anomalous electron configurations


None

Effective Nuclear Charge, Columb’s Law

Effective Nuclear Charge (Zeff)

Sizes of atoms and ions, Bonding Atomic radius (covalent radius)

Periodic Trends in Atomic Radii, Periodic Trends in Ionic Radii, Isoelectric Series, Ionization Engery, Ionization Energy

Variations in Successive Ionization Engergies, Periodic Trends in Ionization Energies

Electron Configurations of Ions, Electron Affinity, Electron Affinity

Metals, Nonmetals, and Metalloids, Metallic Character, Metals

Nonmetals

Metalloids, Trends for Group 1A and 2A metals, Group 1A: Alkali Metals, hydride ion

Group 2A: Alkaline Earth Metals

Trends for Selected Nonmetals, Hydrogen, Group 6A: The Oxygen Group, Ozone

Group 7A: The Halogens

Group 8A: The Noble Gases

Pressure, Pressure (P), Atmospheres and the Barameter

Pascal (Pa), Bar, Standard Atmospheric Pressure, atm, torr


Troublesome Atmosphere Problems

The Gas Laws, The Pressure Volume Relationship (Boyle’s Law), Boyle’s Law

The Temperature Volume Relationship, Charles’ Law, Charels’ LawThe Quantity Volume Relationship, Avogadro’s Law, Avogadro’s Hypothosis

The Ideal Gas Equation

Ideal Gas, Gas Constant (R ), Standard Temperature and Pressure, RElating the Ideal Gas Equation and The Gas Laws

Futher Aplications of the Ideal Gas Equation

None


Tricky Problem

Volumes of Gases in Chemical Reactions, Gas Mixtures and Partial Pressures, Dalton’s Law of Partial Pressures (Partical Pressure).

Partial Pressures and Mole Fractions, Mole fractions, Kinetic Molecular Theory/Theory of Moving Particles

Distributions of Molecular Speed, Root-mean-square (rms, urms) speed

Apply Kinetic Molecular Theory and the Gas Laws, Molecular Effusion and Diffusion

Effusion, Diffusion, Graham’s Law of Diffusion

Diffusion and Mean Free Path, Mean Free Path, Real Gases: Deviations from Ideal Behavior

None
