Physical Properties & Chemical Properties 2019
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Physical Property
can be observed and measured without changing the identity of the substance
Melting Point
temperature at which a substance changes from a solid to a liquid (physical prop)
Boiling Point
temperature at which a substance changes from a liquid to a gas (physical prop)
Density
the relationship between the mass of a material and its volume
D=m/v (physical prop)
Color
by itself, this is not a significant identifier of a substance (physical prop)

Chemical Property
can be observed when substances react (combine) with another substance; as a result it becomes a new substance
Flammability
involves a substance reacting with oxygen quickly to produce light and heat (chemical prop)

Ability to Rust
involves a substance (iron) reacting slowly with oxygen (chemical prop)

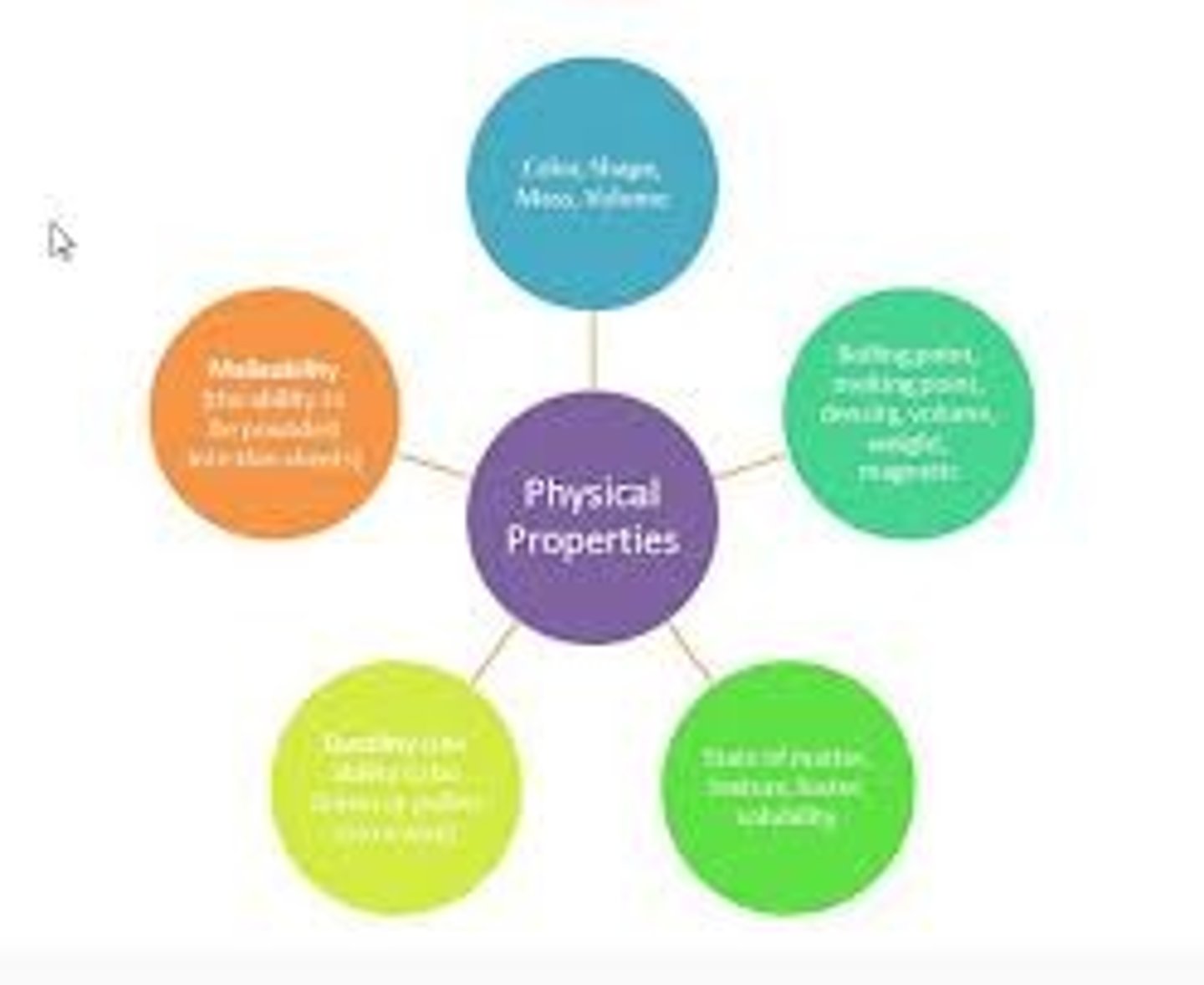
Physical Properties of Matter Examples
Melting Point; Boiling Point; Density; Color, Conductivity, Ductile
Malleable
Chemical Properties of Matter Examples
Flammability; Ability to Rust; Formation of new product
Texture
how the surface of a substance feels (physical prop)

Odor
how a substance affects the olfactory (smell) receptors (physical prop)

Solubility
describes how easily a substance dissolves when mixed with another substance (physical prop)

Reactivity
describes how a substance reacts when exposed to another substance (chemical prop)
Three pieces of evidence a chemical change took place
A change in color, the formation of gas, change in temperature
mixture
A combination of two or more substances that are not chemically combined

element
If all the atoms in a substance have the same identity
chemical property
a characteristic of a substance that indicates whether it can undergo a certain chemical change
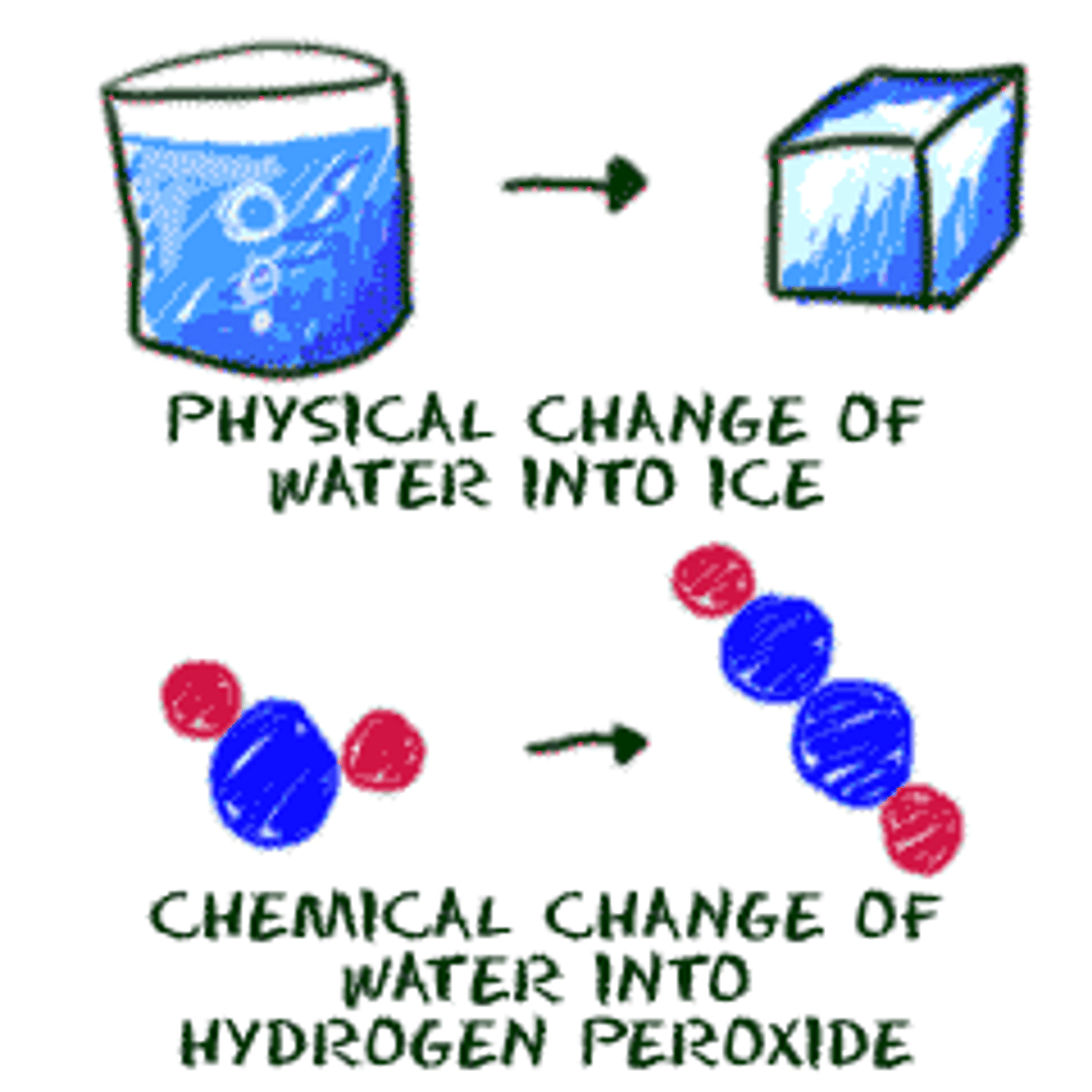
chemical change
a change of one substance to another

physical change
a change in size, shape, or state of matter

physical property
any characteristic of a material that you can observe without changing the identity of the substance that make up the material
compound
a substance in which the atoms of two or more elements are combined in a fixed proportion

pure substance
a type of matter with a fixed composition