🫁 Pulmonary Aspects of Acid‑Base Balance
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
What are normal ABG values?
PaO₂: ~100 mmHg (N: 75–100)
PaCO₂: ~40 mmHg (N: 35–45)
[HCO₃⁻]: ~24 mEq/L
pH: ~7.4 (normal [H⁺] ~40 nEq/L)
↑ pH = alkalosis, ↓ pH = acidosis
Define acidosis vs alkalosis.
Acidosis: pH < 7.35, excess H⁺ → arrhythmias, impaired cardiac function
Alkalosis: pH > 7.45, reduced H⁺ → seizures, neuromuscular irritability, vascular collapse
What are the principal buffers in body fluids?
Blood: Bicarbonate (pK 6.1), Proteins (pK ~7.4), Hemoglobin (pK ~7.4)
Interstitial fluid: Bicarbonate (pK 6.1)
Intracellular fluid: Proteins (~7.0), Phosphate (pK 6.8)
Kidney: Phosphate, Ammonia (NH₃/NH₄⁺)
How do organs buffer H⁺?
Blood: fastest (bicarbonate/protein) → instantaneous
Lungs: regulate CO₂ elimination → minutes–hours
Kidneys: excrete H⁺, reabsorb HCO₃⁻ → hours–days
Bone: exchanges Ca²⁺/phosphate, releases carbonate → hours–days
Ionic shift: H⁺/K⁺ exchange → stabilizes pH in 2–4 hours
What is the Henderson–Hasselbalch equation?
Reaction: H₂O + CO₂ ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻ (carbonic anhydrase)
Equation: pH = pK + log([HCO₃⁻] / (0.03 × PaCO₂))
Key: Bicarbonate = main extracellular buffer; CO₂ controlled by lungs; HCO₃⁻ by kidneys
Interpretation:
• Arterial Blood Gas (ABG) is the primary method for diagnosing acid-base disorders.
• Interpretation involves pH, PaCO₂, and HCO₃⁻ to determine the type of disorder and
compensation status.
Define respiratory acidosis and alkalosis.
Respiratory Acidosis: Hypoventilation → ↑ PaCO₂, ↓ pH; causes: airway obstruction, COPD, CNS depression; compensation: kidneys retain HCO₃⁻
Respiratory Alkalosis: Hyperventilation → ↓ PaCO₂, ↑ pH; causes: anxiety, altitude, fever; compensation: kidneys excrete HCO₃⁻
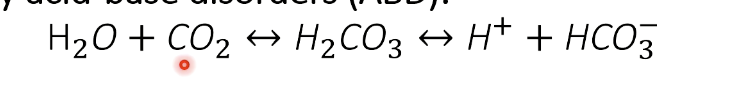
What are causes of respiratory alkalosis?
Central: head injury, stroke, anxiety, pain, drugs (salicylates, analeptics), progesterone, cytokines
Peripheral chemoreceptor stimulation: hypoxemia
Pulmonary: embolism, pneumonia, asthma, pulmonary edema
Iatrogenic: controlled ventilation
MI HY: In other words, to look at that, what may cause to increase ventilation. Abnormally high ventilation that will reduce partial pressure CO2 and will result in alkalosis
What are causes of respiratory acidosis?
Inadequate ventilation: CNS depression (opiates, trauma), neuromuscular disorders (GBS, MG), airway obstruction, COPD, chest trauma, ARDS
Overproduction of CO₂: hypercatabolic states (malignant hyperthermia)
Increased intake of CO₂: rebreathing, added CO₂ in inspired gas
Differentiate acute, chronic, compensated respiratory ABD.
Acute: no compensation, pH deviates (e.g., narcotic overdose)
Chronic: partial renal compensation, pH moves toward normal but not fully corrected
Compensated: pH ≈ 7.4, but PaCO₂ and HCO₃⁻ abnormal
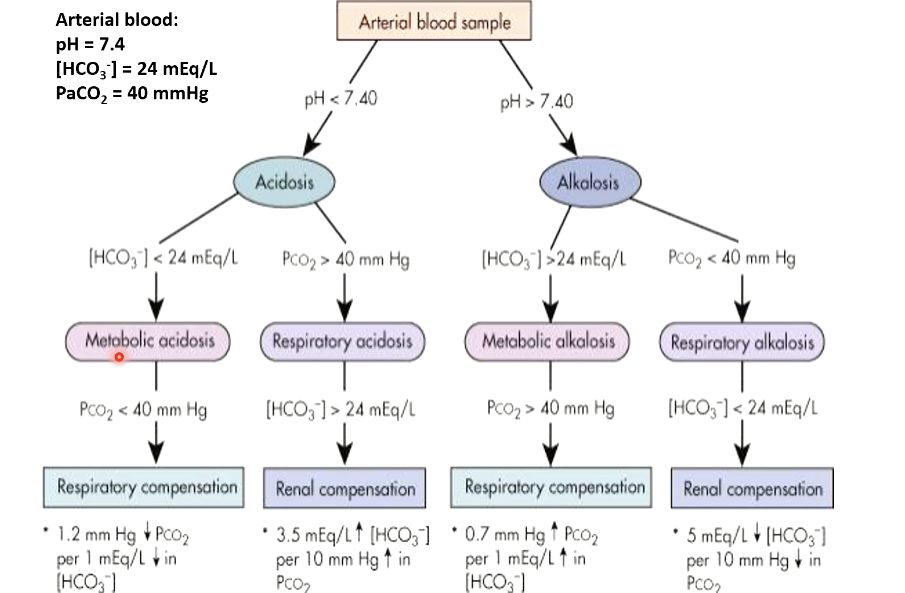
What is the stepwise approach to ABD diagnosis?
Check pH: <7.40 = acidosis; >7.40 = alkalosis
Check PaCO₂:
↑ PaCO₂ + ↓ pH = respiratory acidosis
↓ PaCO₂ + ↑ pH = respiratory alkalosis
If PaCO₂ doesn’t match pH → metabolic disorder (Compensation evaluation covered in renal physiology)
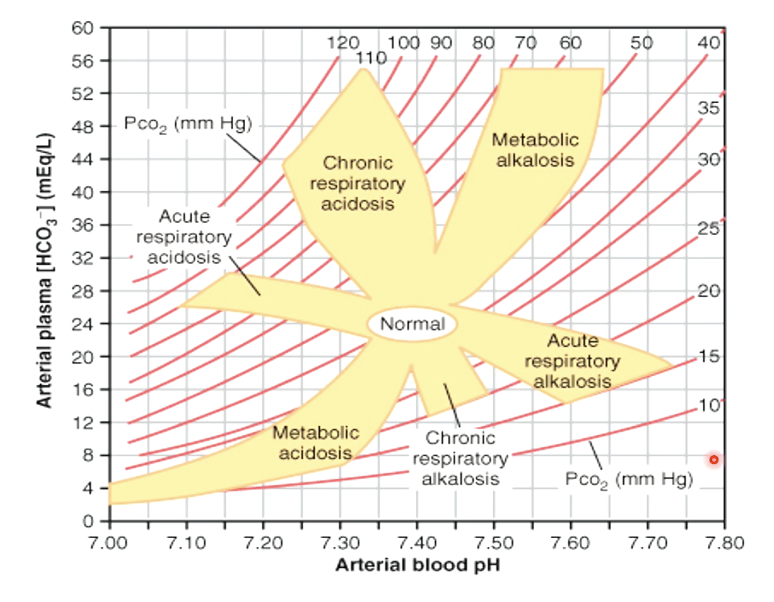
How is a nomogram used in ABD diagnosis?
Plot pH (X‑axis) vs [HCO₃⁻] (Y‑axis) with PaCO₂ lines
Identify zone: respiratory acidosis/alkalosis, metabolic acidosis/alkalosis
Example: pH 7.25, PaCO₂ 55, HCO₃⁻ 24 → acute respiratory acidosis
What are the key points of pulmonary aspects of ABD?
Normal ABG: pH 7.4, PaCO₂ 40, HCO₃⁻ 24
Respiratory acidosis: ↓ pH, ↑ PaCO₂ (hypoventilation)
Respiratory alkalosis: ↑ pH, ↓ PaCO₂ (hyperventilation)
Diagnosis: pH first, then PaCO₂ → respiratory vs metabolic
Compensation: respiratory (fast), renal (slow)
Tools: Henderson–Hasselbalch, flowchart, nomogram
PaCO₂ 52 mmHg, pH 7.3 → ?
Respiratory acidosis
PaCO₂ 52 mmHg, pH 7.5 → ?
Respiratory alkalosis
[HCO₃⁻] 35 mEq/L → ?
Renal compensation for respiratory acidosis
ABG pH 7.25, PaCO₂ 55, HCO₃⁻ 24 → ?
Acute respiratory acidosis